Maybe during your science class, you had cerebral pain with molecular and structural formulas. Indeed, even I had experienced a similar issue.
Molecular and structural formulas manage particles and particles. We realize that particles are framed when molecules are joined by and large.
Key Takeaways
- A molecular formula shows the types and number of atoms in a molecule, while a structural formula shows the arrangement of atoms in the molecule.
- The molecular formula is a simplified representation of the compound, while the structural formula provides a detailed and comprehensive view.
- The molecular formula is used to describe the chemical composition of a substance, while the structural formula is used to illustrate how the atoms are arranged within the molecule.
Molecular vs Structural Formula
The molecular formula gives the number and type of their atoms. The structural formula shows the arrangement and bonds of atoms. The molecular formula is a simple representation of a compound, while the structural formula provides a detailed representation of the compound’s structure.
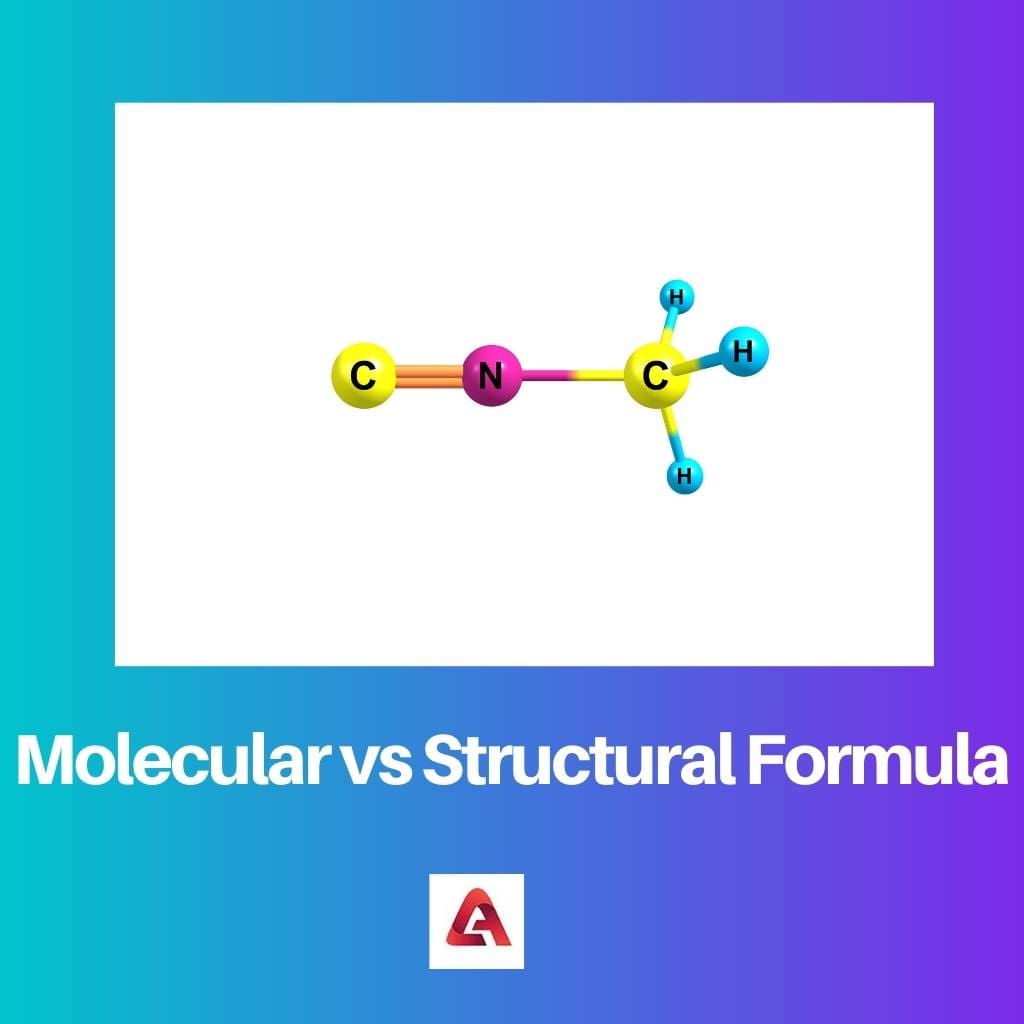
The molecular formula or substance formula of a compound is the portrayal of the kinds of particles and their proportions present in that compound.
This primary equation gives numerous insights concerning the atom, and the characteristics of the compound can additionally be anticipated utilizing these subtleties.
Comparison Table
| Parameters of Comparison | Molecular Formula | Structural Formula |
|---|---|---|
| Definition | The molecular formula of a molecule, also known as its substance formula, shows the various sorts of iotas and their amounts. | The structural formula is used to indicate not only how many atoms are present but also how they are arranged spatially. |
| Proportion | The number of molecules in the chemical is expressed as a percentage. | The structural formula specifies the activity of iotas as well as the general locations of the compound’s helpful groupings. |
| Uses | It’s widely used to categorize basic atoms, identify whether a compound is a binary, ternary, quaternary, or multi-component complex, and so on. | The structural formula may be used to define complicated atoms and to predict mixed substance qualities (such as extreme) as well as actual properties (like the edge of boiling over). |
| Arrangement | A molecular formula specifies the number of atoms as well as their spatial arrangement. | The number of molecules and their sequence of activity in space are represented by a structural formula. |
| Trace | The molecular formula can be traced on a molecular level. | The structural formula can be traced on a structural level. |
What is Molecular Formula?
A molecular formula is one of the least difficult approaches to communicating the composition of complex particles. With a molecular formula, it can determine the genuine number of particles of every component in a particle.
When composing the molecular formula, you need to compose the images for every one of the components that are contained inside a particle.
That would be H2O. When there is just a single particle of a specific component, the number “1” shouldn’t be written in the molecular formula.
Building the molecular formula appears to be really simple. However long you know the image for every component and the number of iotas there are in a specific atom, you’ll never turn out badly.
What is Structural Formula?
Surrounding us, we have Chemical mixtures – they are in our refreshments, food, and things one uses in the everyday. We can recognize these synthetic mixtures by their sub-atomic equations.
We have no clue about which molecule of a component is connected to which. This is where the molecular formula emerges.
The electron dab primary equation portrayal utilizes spots to mean the electrons engaged with the holding in the midst of various molecules.
The consolidated equation actually utilizes lines among fortified iotas however is a tranquil and more modest technique to define the boundary bond primary recipe since it discards the carbon and hydrogen bonds.
Main Differences Between Molecular and Structural Formula
- A molecular formula specifies the number of atoms as well as their spatial arrangement, whereas the number of molecules and their sequence of activity in space are represented by a structural formula.
- The molecular formula can be traced on a molecular level, whereas the structural formula can be traced on a structural level.
- https://www.nature.com/articles/nchem.1422
- https://www.ingentaconnect.com/contentone/asp/asl/2018/00000024/00000006/art00053

Thank you for the detailed explanation. I’m glad to have a clearer understanding of the differences between molecular and structural formulas.
Indeed, the distinction is quite intriguing. It provides a better comprehension of chemical compositions.
The references provided have further enriched the knowledge shared in the article, contributing to its credibility.
Absolutely. The well-incorporated references add substantial value to the content.
The description of the molecular and structural formulas is extensive and comprehensive, providing a holistic view of the topic.
Indeed, the content has certainly expanded my understanding of these formulas.
Agreed! The depth of information covered is truly commendable.
The detailed explanation has offered valuable insights into the uses of both molecular and structural formulas.
Absolutely. It’s commendable how the author has presented the content in such an informative manner.
An excellent summary of the differences. The table comparison is especially helpful in contextualizing the information.
Definitely. The tabulated parameters truly highlight the distinctions between the two formulas.
I couldn’t agree more. The tabulated data makes the details more accessible.
The explanation of the molecular and structural formulas, along with the differences between the two, is articulated exceptionally well.
I couldn’t agree more. The author has achieved clarity and coherence in the delivery of information.
Indeed. The writing style is on point, and it significantly enhances the understanding of the content.
The detailed comparisons and explanations are invaluable for understanding the distinctions between the molecular and structural formulas.
Absolutely. The article’s content has expanded the depth of my understanding in this area.
Definitely, the detailed analyses have enhanced my familiarity with molecular and structural formulas.
The content has indeed deepened my knowledge of molecular and structural formulas.
This is a well-written piece on molecular and structural formulas and their respective uses. The references are also quite solid.
Absolutely, it has certainly enhanced my knowledge in this area.
Agreed! The comparison between the two formulas is thoroughly enlightening.
The section on molecular and structural formulas is presented in a clear and articulate manner, making it easier to comprehend.
Absolutely. The lucidity of the content simplifies the complex nature of these formulas.