Los compuestos iónicos se forman mediante la transferencia de electrones entre átomos, lo que da como resultado iones cargados que se mantienen unidos por fuerzas electrostáticas. Los compuestos moleculares, por otro lado, están compuestos de átomos unidos covalentemente, que comparten electrones para formar moléculas discretas.
Puntos clave
- Los compuestos iónicos están formados por iones que se mantienen unidos por fuerzas electrostáticas.
- Los compuestos moleculares están formados por moléculas que se mantienen unidas por enlaces covalentes.
- Los compuestos iónicos tienen puntos de fusión y ebullición más altos que los compuestos moleculares y son solubles en agua.
Compuestos Iónicos vs Compuestos Moleculares
Los compuestos iónicos se forman por los enlaces iónicos en los que los átomos se atraen electrostáticamente entre sí. Tienen interacción de cationes y aniones en ellos. Mientras que los compuestos moleculares están formados por enlaces covalentes, en los cuales los electrones son compartidos por los átomos formando el enlace.
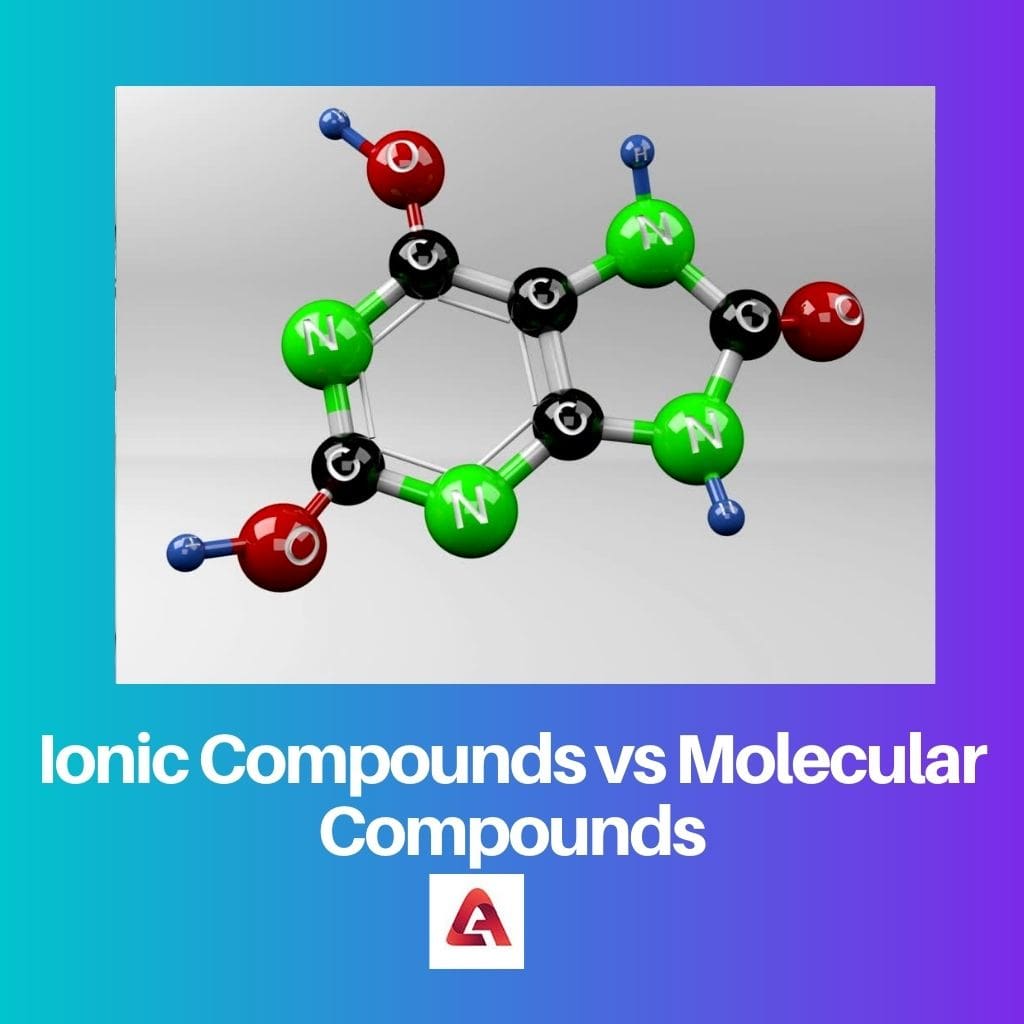
Para comprender mejor la diferencia, debe comprender bien la terminología básica. Dos o más dos átomos de diferentes elementos se combinan para formar una molécula, que es la unidad básica de un compuesto.
Cada compuesto es diferente en términos de propiedades. Esto se debe al hecho de que cada elementos que un compuesto consta de posee diferentes propiedades. La electronegatividad es también uno de los términos más importantes que debe conocer.
La electronegatividad es la tendencia de un átomo de un elemento a atraer los electrones de otros elementos hacia su núcleo. Un compuesto puede ser polar o no polar, y esto depende enteramente de la electronegatividad de los elementos.
Tabla de comparación
| Feature | Compuestos ionicos | Compuestos moleculares |
|---|---|---|
| Espiritual | Formado por el transferencia de electrones entre un metal y un no metal, lo que resulta en iones con cargas opuestas (cationes y aniones) que se atraen entre sí. | Formado por el intercambio de electrones entre dos o más no metales, formando enlaces covalentes para mantener unidos los átomos. |
| Tipo de unión | Enlace iónico (atracción electrostática entre iones con cargas opuestas) | Unión covalente (compartición de electrones entre átomos) |
| Estructura | Estructura reticular cristalina, con disposición regular de cationes y aniones. | Moléculas discretas, con formas y disposiciones específicas de los átomos. |
| Estado a temperatura ambiente | Típicamente sólidos | Puede ser sólidos, líquidos o gases dependiendo del compuesto. |
| Conductividad eléctrica | Buenos conductores en estado fundido o acuoso, ya que los iones pueden moverse libremente. | malos conductores en todos los estados, ya que los electrones están estrechamente unidos dentro de las moléculas. |
| Solubilidad en agua | Generalmente soluble en agua debido a la atracción de iones a las moléculas de agua. | Solubilidad variable en agua, dependiendo de la polaridad y el tamaño de la molécula. |
| Ejemplos | Cloruro de sodio (NaCl), Óxido de calcio (CaO), Sulfato de potasio (K₂SO₄) | Agua (H₂O), Dióxido de carbono (CO₂), Metano (CH₄) |
¿Qué son los compuestos iónicos?
Los compuestos iónicos son un tipo de compuesto químico caracterizado por la presencia de iones, que son átomos o grupos de átomos que han ganado o perdido electrones, dando como resultado una carga eléctrica neta. Estos compuestos normalmente se forman cuando los átomos de los metales reaccionan con los átomos de los no metales, lo que lleva a la transferencia de electrones del metal al no metal.
Formación de compuestos iónicos
La formación de compuestos iónicos implica el proceso de ionización, donde los átomos ganan o pierden electrones para lograr una configuración electrónica estable. Normalmente, los metales tienden a perder electrones para formar iones cargados positivamente conocidos como cationes, mientras que los no metales tienden a ganar electrones para formar iones cargados negativamente llamados aniones.
Por ejemplo, en la formación de cloruro de sodio (NaCl), los átomos de sodio (Na), con un electrón en su capa más externa, pierden este electrón para lograr una configuración electrónica estable del neón, formando iones Na⁺. Por el contrario, los átomos de cloro (Cl), que requieren un electrón para completar su capa más externa, ganan este electrón para formar iones Cl⁻. La atracción resultante entre iones con cargas opuestas conduce a la formación de un enlace iónico.
Características de los compuestos iónicos
- Estructura de celosía cristalina: Los compuestos iónicos suelen formar una estructura reticular tridimensional, donde cada catión está rodeado por aniones y viceversa. Esta disposición maximiza la atracción entre iones con cargas opuestas, lo que da como resultado fuertes fuerzas electrostáticas que mantienen unida la red.
- Altos puntos de fusión y ebullición: Debido a las fuertes fuerzas electrostáticas entre los iones, los compuestos iónicos generalmente tienen puntos de fusión y ebullición altos. Esto se debe a que se requiere una cantidad considerable de energía para vencer estas fuerzas y romper los enlaces que mantienen unida la red.
- Solubilidad en agua: Muchos compuestos iónicos son solubles en agua debido a la naturaleza polar de las moléculas de agua. Cuando un compuesto iónico se disuelve en agua, las moléculas de agua rodean los iones individuales, separándolos efectivamente de la red cristalina y permitiéndoles dispersarse por toda la solución.
- Conductividad: En estado sólido, los compuestos iónicos no conducen electricidad porque los iones se mantienen en posiciones fijas dentro de la estructura reticular. Sin embargo, cuando se disuelven en agua o se funden, los iones quedan libres para moverse y pueden conducir electricidad, lo que hace que los compuestos iónicos fundidos y sus soluciones acuosas sean buenos conductores de electricidad.
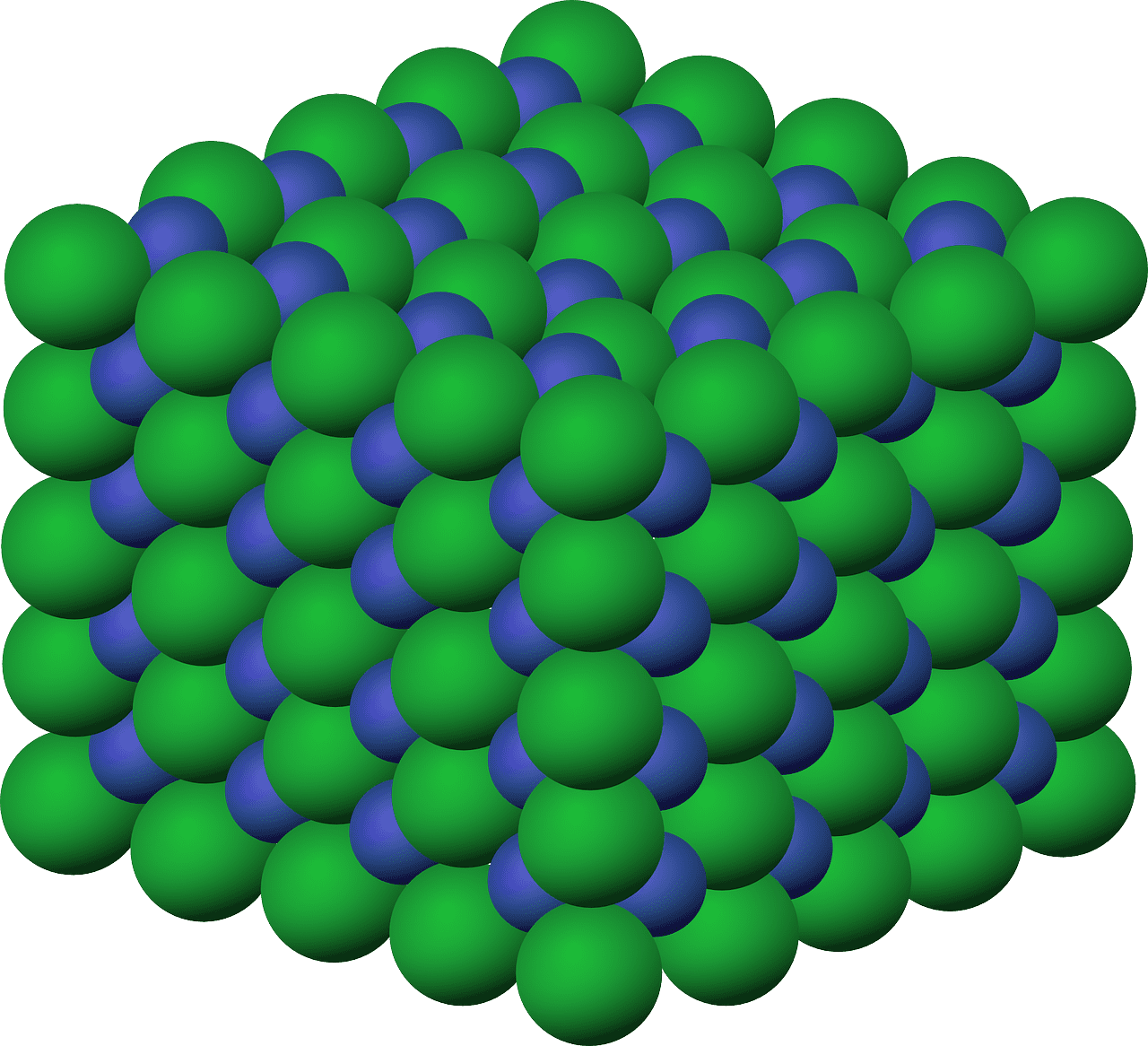
¿Qué son los compuestos moleculares?
Los compuestos moleculares son compuestos químicos compuestos de moléculas formadas al compartir electrones entre átomos, principalmente a través de enlaces covalentes. A diferencia de los compuestos iónicos, que implican la transferencia de electrones que conducen a la formación de iones, los compuestos moleculares constan de unidades discretas llamadas moléculas, donde los átomos se mantienen unidos mediante pares de electrones compartidos.
Formación de compuestos moleculares
Los compuestos moleculares se forman cuando los átomos de los no metales se unen compartiendo electrones para lograr una configuración electrónica estable. En un enlace covalente, los átomos comparten uno o más pares de electrones, lo que da como resultado la formación de una molécula. Compartir electrones permite que cada átomo alcance una capa exterior completa, que normalmente consta de ocho electrones (regla del octeto), o dos electrones en el caso del hidrógeno.
Por ejemplo, en la formación de agua (H₂O), dos átomos de hidrógeno (H) comparten cada uno un par de electrones con un átomo de oxígeno (O). Este intercambio de electrones crea enlaces covalentes entre los átomos de hidrógeno y oxígeno, lo que da como resultado la formación de una molécula de agua.
Características de los compuestos moleculares
- Puntos de fusión y ebullición bajos: Los compuestos moleculares generalmente tienen puntos de fusión y ebullición más bajos en comparación con los compuestos iónicos. Esto se debe a que las fuerzas intermoleculares entre moléculas (como las fuerzas de van der Waals o los enlaces de hidrógeno) son más débiles que los enlaces iónicos presentes en los compuestos iónicos.
- Solubilidad variada: La solubilidad de los compuestos moleculares en agua varía según la polaridad de las moléculas. Las moléculas polares tienden a disolverse en disolventes polares como el agua, mientras que las moléculas no polares se disuelven mejor en disolventes no polares. Este comportamiento de solubilidad se debe a las interacciones entre las regiones polares o no polares de las moléculas y las moléculas del disolvente.
- Existencia en múltiples fases: Los compuestos moleculares pueden existir en diferentes fases (sólido, líquido o gaseoso) en condiciones estándar, dependiendo de factores como el tamaño molecular, la forma y las fuerzas intermoleculares. Por ejemplo, algunos compuestos moleculares, como el agua, pueden existir en las tres fases dependiendo de la temperatura y la presión.
- No conductividad: Los compuestos moleculares generalmente no conducen la electricidad en ningún estado (sólido, líquido o gaseoso) porque no contienen iones libres ni partículas cargadas móviles. La corriente eléctrica requiere la presencia de partículas cargadas, que están ausentes en los compuestos moleculares donde los electrones se comparten en lugar de transferirse.
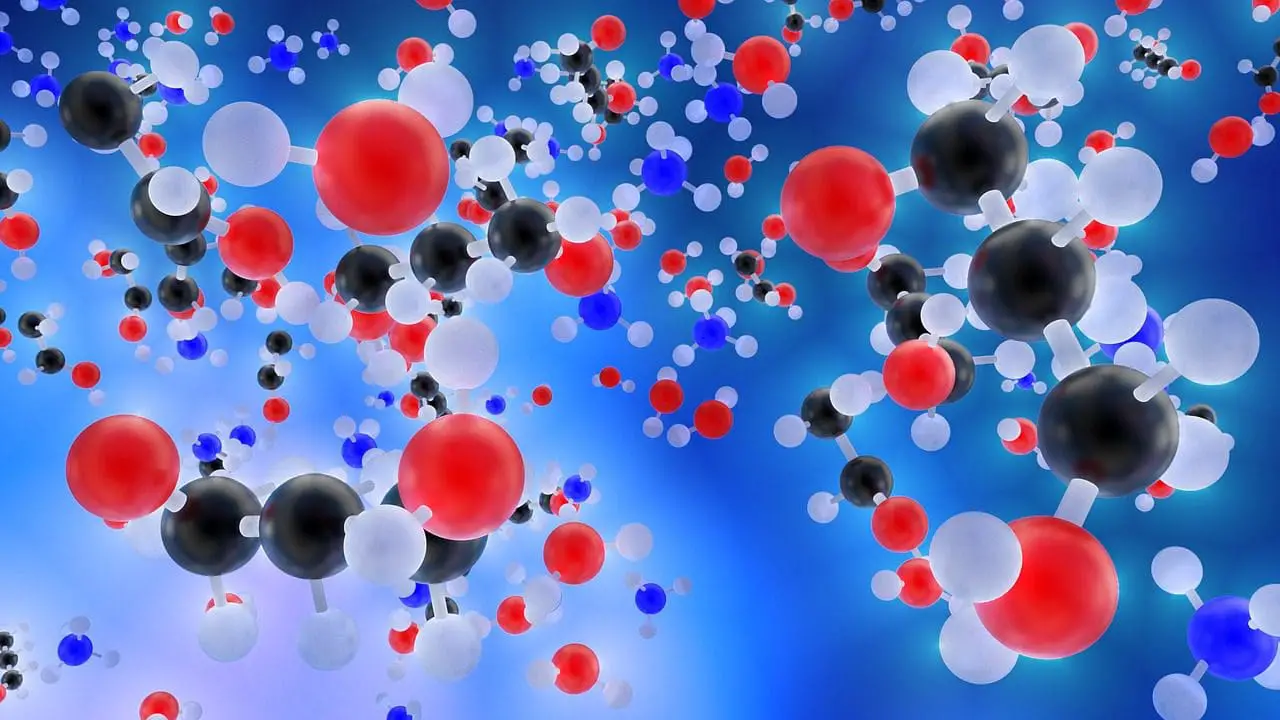
Principales diferencias entre compuestos iónicos y compuestos moleculares
- Mecanismo de vinculación:
- Los compuestos iónicos se forman mediante la transferencia de electrones, lo que resulta en la formación de iones y atracción electrostática entre iones con cargas opuestas.
- Los compuestos moleculares se forman al compartir electrones entre átomos, lo que da como resultado la formación de moléculas discretas unidas por enlaces covalentes.
- Composición:
- Los compuestos iónicos están compuestos de iones, que son átomos o grupos de átomos con una carga eléctrica neta.
- Los compuestos moleculares están compuestos de moléculas, que son grupos de átomos unidos por enlaces covalentes.
- Propiedades físicas:
- Los compuestos iónicos suelen tener puntos de fusión y ebullición elevados debido a las fuertes fuerzas electrostáticas entre los iones.
- Los compuestos moleculares suelen tener puntos de fusión y ebullición más bajos en comparación con los compuestos iónicos debido a fuerzas intermoleculares más débiles entre las moléculas.
- Conductividad:
- Los compuestos iónicos conducen la electricidad cuando se disuelven en agua o se funden debido a la presencia de iones libres capaces de transportar carga eléctrica.
- Los compuestos moleculares generalmente no conducen electricidad en ningún estado (sólido, líquido o gaseoso) porque no contienen iones libres ni partículas cargadas móviles.
- Solubilidad:
- Muchos compuestos iónicos son solubles en agua debido a la naturaleza polar de las moléculas de agua, que pueden rodear y disociar los iones de la red cristalina.
- La solubilidad de los compuestos moleculares varía según la polaridad de las moléculas: las moléculas polares se disuelven en disolventes polares y las moléculas no polares se disuelven en disolventes no polares.

Última actualización: 06 de marzo de 2024

Piyush Yadav ha pasado los últimos 25 años trabajando como físico en la comunidad local. Es un físico apasionado por hacer que la ciencia sea más accesible para nuestros lectores. Tiene una licenciatura en Ciencias Naturales y un Diploma de Postgrado en Ciencias Ambientales. Puedes leer más sobre él en su página de biografía.

Ojalá se enseñara química así en la escuela, haría que la materia fuera mucho más fácil de entender.
Absolutamente, esta fue una gran lectura.
Estoy de acuerdo, las comparaciones y explicaciones fueron realmente útiles.
El artículo cubre el tema en profundidad y proporciona una descripción general completa de los compuestos iónicos y moleculares.
Bien articulado y basado en investigaciones, un excelente trabajo.
Las comparaciones entre compuestos iónicos y moleculares son muy esclarecedoras.
El artículo proporciona una descripción equilibrada y bien elaborada de los compuestos iónicos y moleculares.
El contenido está cuidadosamente presentado y minuciosamente investigado, ¡excelente trabajo!
La comparación detallada de las propiedades y características de los compuestos iónicos y moleculares es a la vez esclarecedora y atractiva.
El artículo proporciona un análisis claro y sistemático de compuestos iónicos y moleculares.
Sí, la tabla comparativa es muy útil para resaltar las distinciones entre los dos tipos de compuestos.
La información presentada aquí me parece bastante útil para comprender las diferencias fundamentales entre compuestos iónicos y moleculares.
El contenido está bien explicado, lo que lo hace accesible a una amplia gama de lectores.
Absolutamente, el artículo es un gran recurso para los estudiantes de química.
Una comparación completa. Muy bien estructurado e informativo.
No podría haberlo explicado mejor, ¡excelente trabajo!
Las explicaciones son claras y concisas, lo que facilita la comprensión de los conceptos presentados.
La descripción de las propiedades y diferencias entre compuestos iónicos y moleculares es excelente.
Un artículo muy informativo, ¡bien hecho!
Esta fue una explicación muy educativa, ¡gracias!
Estoy de acuerdo, los ejemplos de compuestos iónicos y moleculares ayudan a solidificar los conceptos.
¡Excelente contenido, bien resumido!
La explicación de la electronegatividad en relación con compuestos iónicos y moleculares es particularmente reveladora.
Por supuesto, comprender la electronegatividad es fundamental para comprender los enlaces químicos.
Estoy totalmente en desacuerdo con algunos de los puntos planteados en este artículo, particularmente la discusión sobre los puntos de ebullición y fusión.