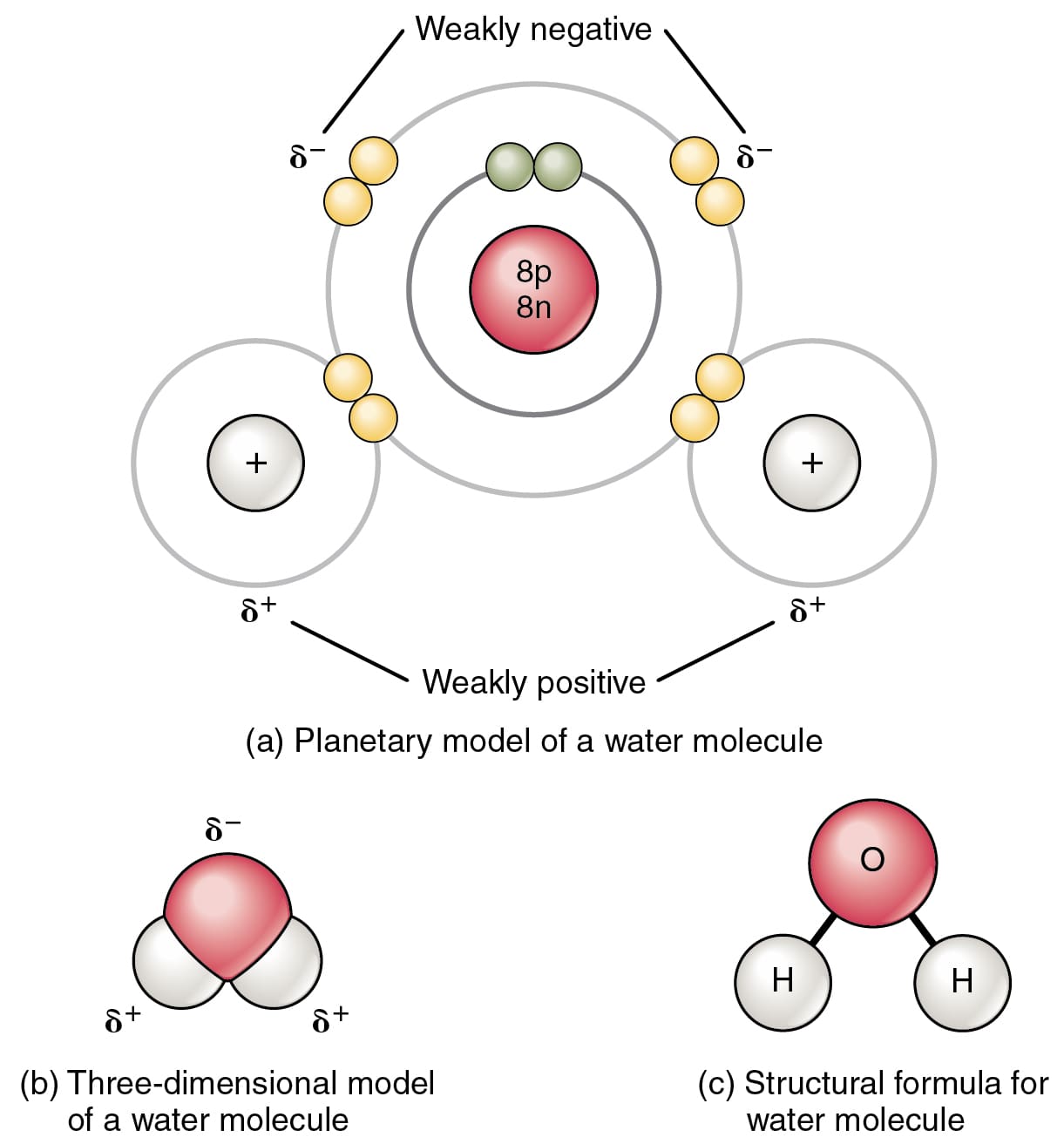
There are so many things in nature that bond with one another to form a new structure or a new class of elements. For example: Consider water, how is it formed?
It is formed by the process of chemical bonding in which 2 atoms of Hydrogen and 1 atom of Oxygen bonds to form the liquid state of water. Chemical bonding is important for living organisms and the proper functioning of their body parts.
Key Takeaways
- Ionic bonds form between metal and nonmetal elements through electron transfer, while covalent bonds form between nonmetals through electron sharing.
- Metallic bonds occur between metal atoms, involving a sea of delocalized electrons.
- Ionic compounds have high melting and boiling points. Covalent compounds have lower melting and boiling points, and metallic compounds have varying melting and boiling points.
Ionic vs Covalent vs Metallic Bonds
Ionic bonds are formed between ions with opposite charges. These ions can be formed by the transfer of one or more electrons from one atom to another. Covalent bonds are formed when atoms share one or more pairs of electrons. This type of bond occurs between two non-metal atoms. Metallic bonds are formed between atoms of metals. In a metallic bond, the valence electrons are not localized on a particular atom, but rather are free to move throughout the entire metal lattice.

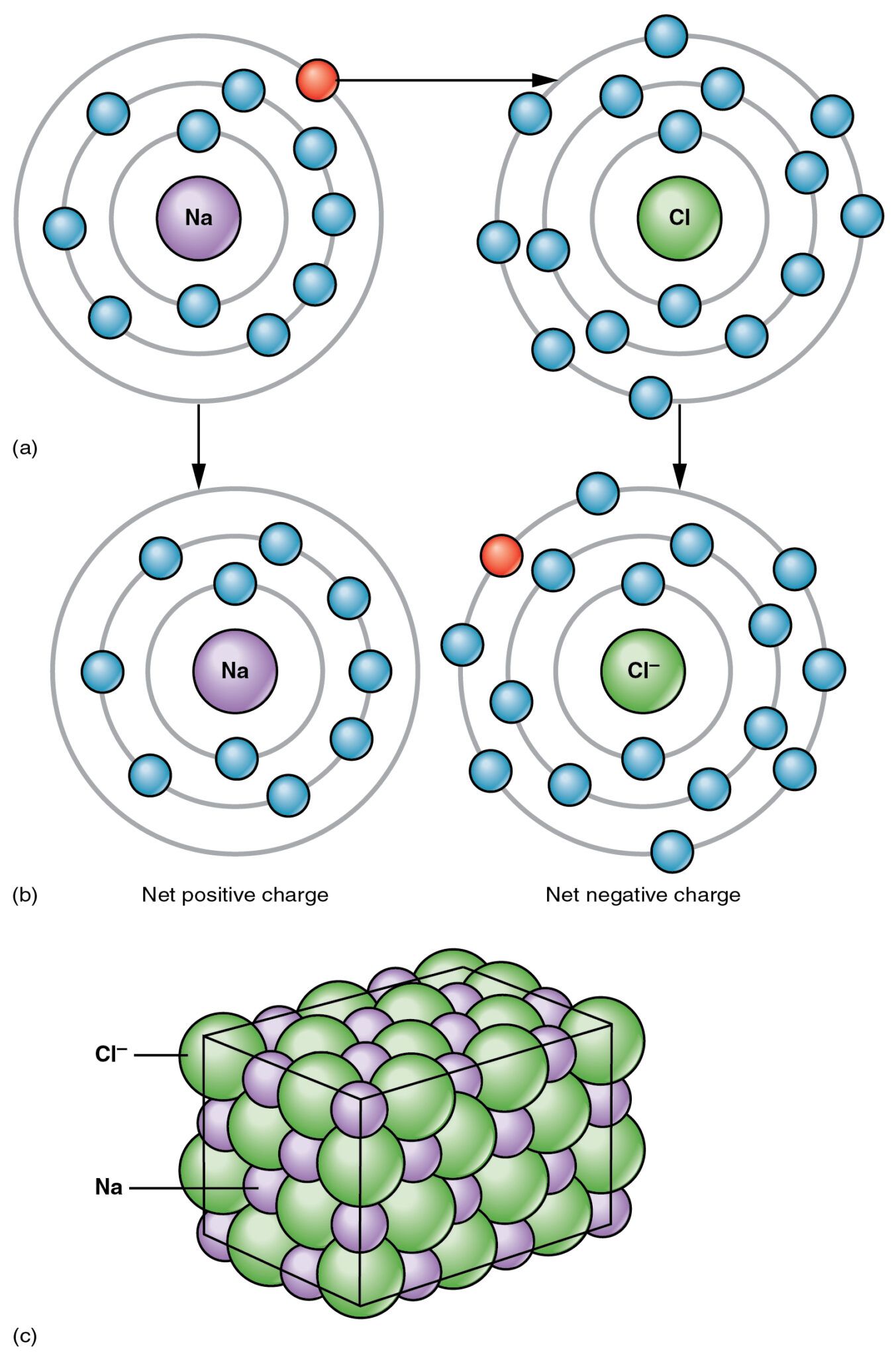
Ionic Bond is the transfer of electrons from a metallic atom to a non-metallic atom, for example, consider Sodium Chloride; Sodium’s atomic number is 11 and electronic configuration is 2,8,1 Sodium has an extra electron in its outermost shell.
On the other hand, Chlorine’s atomic number is 17 and electronic configuration is 2,8,7 Chlorine has 7 electrons in its outermost shell so Chlorine needs one more electron to complete its outmost shell so all the atoms will be stable.
As Sodium has an extra electron when Sodium reacts with Chorine, Sodium donates that extra electron to Chorine. In this process, Sodium becomes positively charged ions (Na+ ) and after accepting the electron Chorine becomes negatively charged ions (Cl–).
These two oppositely charged ions are held by a strong electrostatic force of attraction known as the Ionic Bond.
A Covalent Bond is formed when two or more atoms join together by mutual sharing of electrons. For example, considering the formation of Oxygen gas (O2) we will take 2 atoms of oxygen as we know the number of the electron in Oxygen is 8 and there are 6 valence electrons in the outer shell.
When these two outer valence shells that contain 6 electrons of oxygen get 2 more electrons it will be stable, as its Octet Rule will be fulfilled.
To stabilize themselves these two atoms of Oxygen combine and share two atoms of Oxygen mutually to form Oxygen gas, this is called Covalent Bond.
Metallic Bonding is the electrostatic attraction between metal ions arranged in a lattice (regular repeating pattern) structure and the free-floating of electrons around them.
In a metallic structure, there are only metal ions these metal ions are arranged side by side in a regular repeating pattern, and the free-floating electrons act as glue and hold the structure in place.
This is a very strong attraction hence the melting point and boiling point of metals is very high. Free-floating electrons are the reasons that metal is a good conductor of electricity.
Comparison Table
| Parameters of Comparison | Ionic Bond | Covalent Bond | Metallic Bond |
|---|---|---|---|
| Formation | Metal and non-metal | Two non-metal | Positively Charges atoms and free-floating electrons |
| Polarity | High | Low | Non-polar |
| Shape of Molecule | No definite shape, lattice structure | Definite Shape | No definite shape |
| Examples | Sodium Chloride, Sulphuric Acid | Methane, Hydro Choric Acid | Sodium, Magnesium |
| Melting point | High melting point | Low melting Point | High Melting Point |
What is Ionic Bond?
Ionic bonding takes place when there is gaining and losing of electrons between a metal and a non-metal, for an Ionic bonding to take place there should be at least one metal.
For example, a Magnesium atom lost two electrons and formed Magnesium ions that are positively charged and it is metal ions; on the other hand, the Oxygen atom gains two electrons and becomes a negatively charged oxide ions and it is a non-metal.
These two atoms attract each other as one is positive and the other is negative and this attraction is called Ionic Bonding.
What is Covalent Bond?
Covalent bonding takes place only in non-metal it can never take place with the metals, it occurs when two or more than two non-metals share a pair of electrons mutually.
For example, we have two Chlorine atoms and the outer shells of these Chlorine atoms overlap and share a pair of an electron to form an element.
The two atoms are joined together which forms a very strong bond that holds them together. This type of bond is called Covalent Bonding.
What is a Metallic Bond?
Metallic bonding occurs between metals, the metals lose electrons to form positive ions and the loose electrons become delocalized electrons which means that they are no longer attached to any ions and float around in a sea of delocalized electrons.
As the ions are positive and the delocalized electrons are negative there will be an attraction between them. The electrostatic attraction between positive ions and negative delocalized electrons is called Metallic Bonding.
Main Differences Between Ionic, Covalent, and Metallic Bonds
- In Ionic Bond one atom provides electrons to another, in Covalent bond two atoms exchange valence electrons, whereas in a Metallic bond metal lattice comprises atoms that share several electrons.
- In Ionic Bond energy of a bond is higher than that of a metallic bond, the same goes with Covalent Bond, whereas in Metallic Bond there is less bond energy than in other primary bonds.
- Ionic Bond is a weak conductor on the other hand Covalent Bond is a very weak conductor, whereas Metallic Bond is a strong conductor.
- Ionic Bond can be in the solid-state, Covalent Bond can be in solid, liquid, and gasses state, whereas Metallic bonds can only be in the solid-state.
- Ionic Bond does not have a malleable trait same goes with Covalent Bond, whereas Metallic Bond has a malleable trait



