The main essence of a combustion reaction is that it involves O-oxygen and is an exothermic reaction. Combustion reactions release energy in the form of heat and light.
An example of a combustion reaction involving Propane and oxygen is as below:
C3H8(g)+5O2(g)→3CO2(g)+4H2O(g)
Combustion reactions can be of two types depending on the amount of oxygen available.
Key Takeaways
- Complete combustion occurs when a fuel burns completely in the presence of an adequate supply of oxygen, producing carbon dioxide and water as byproducts; incomplete combustion occurs when the oxygen supply is insufficient, leading to the formation of carbon monoxide, soot, or other harmful byproducts.
- Complete combustion releases more energy than incomplete combustion, making it more efficient.
- Incomplete combustion can have negative consequences, such as increased air pollution and potential health risks due to carbon monoxide exposure.
Complete Combustion vs. Incomplete Combustion
Complete Combustion and Incomplete Combustion differ in the amount of oxygen available. If the amount is sufficient or more, it is a Complete Combustion reaction; if it is less, it is an Incomplete Combustion reaction.

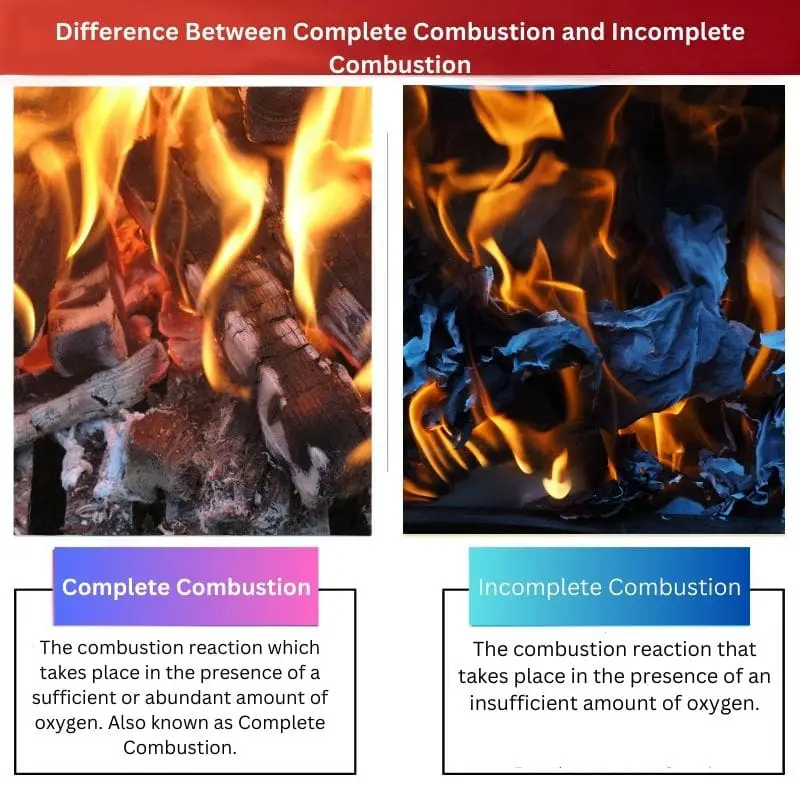
When there is a sufficient or abundant amount of oxygen available during the process of combustion, then the reaction is known as a Complete Combustion reaction.
When the amount of oxygen is insufficient for the process of combustion, then the reaction is known as an Incomplete Combustion reaction.
Comparison Table
| Parameters of Comparison | Complete Combustion | Incomplete Combustion |
|---|---|---|
| Definition | The combustion reaction occurs in the presence of a sufficient or abundant amount of oxygen. Also known as Complete Combustion. | The combustion reaction takes place in the presence of an insufficient amount of oxygen. |
| Flame-type | Blue | Yellow |
| Smoke-type | No smoke | Sooty |
| Products | Usually produces CO2 (Carbon Dioxide) as a primary product. | Usually produces CO (Carbon Monoxide) as a primary product. |
| Energy Production | Produces more energy as compared to Incomplete Combustion when combusting the same reactants. | Produces lesser energy when compared to Complete Combustion when combusting the same reactants. |
What is Complete Combustion?
Complete combustion is the combustion process where the amount of oxygen involved in the relationship is sufficient or more than required.
Usually, these reactions occur with hydrocarbons being on the reactant side as reducing agents. Hydrocarbons and oxygen react together to form water and carbon dioxide.
This is the reason whenever we burn stuff like wood, paper, and similar other items which contain hydrocarbons in them, we see a yellow flame, which is a sign of Incomplete Combustion rather than a Complete Combustion reaction.
A few examples of Complete Combustion reactions are given below:
Complete Combustion of methane:
CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)
Methane is the reducing agent that reacts with oxygen, the oxidizing agent. This gives us carbon dioxide and Hydrogen as the final products. That is the minimum amount of oxygen needed by methane.
Complete Combustion of methanol:
2CH3OH(g) + 3O2(g) → 2CO2(g) + 4H2O(g)
Like the above example, methanol is also the reducing agent, and oxygen is the oxidizing agent. Methanol is a more complex hydrocarbon that requires more oxygen molecules and produces more water and carbon dioxide.
The complete Combustion of a substance gives the most energy possible, which can be extracted. This is because all of the substance is successfully combusted.
This type of combustion is also called ‘clean combustion’ as the products formed by this combustion do not pollute or harm the environment since it is just carbon dioxide and water.
A common example of clean combustion is the burning of LPG in our homes, as it produces a clear blue flame and no smoke.

What is Incomplete Combustion?
An Incomplete Combustion reaction is a reaction where the amount of oxygen present in the reaction is insufficient than the required amount of oxygen needed to carry out the reaction in a complete manner.
Like Complete Combustion reactions, the reactants play the same role: oxygen is an oxidizing agent, and the hydrocarbons are reducing agents.
This type of reaction is undesirable as it releases very little energy compared to complete reactions of the same substances.
A yellow flame with sooty smoke characterizes this reaction. The primary products of this reaction are water and carbon monoxide (CO).
When household appliances get burnt or catch fire, they undergo an Incomplete Combustion reaction. The toxic carbon monoxide hence produced is colorless and odorless.
Some reaction examples of Incomplete Combustion are:
Incomplete Combustion of Propane-LPG
2 C3H8 + 9 O2 → 4 CO2 + 2 CO + 8 H2O + Heat
Some people might state that there is carbon dioxide on the product side, and hence this will be a Complete Combustion reaction.
A very common example of Incomplete Combustion is the burning of coal. This produces a lot of soot and smoke and hence causes a lot of environmental degradation.

Main Differences Between Complete Combustion and Incomplete Combustion
- The products of a complete combustion reaction are environment friendly and do not cause pollution, whereas the products of an Incomplete Combustion reaction are major pollutants in today’s world.
- A complete combustion reaction produces more energy than an Incomplete Combustion reaction with the same product.

The article presents a comprehensive overview of complete and incomplete combustion, along with clear examples. It’s an excellent read for those interested in chemistry.
I completely agree, Anthony29. The article effectively explains the key points and differences between complete and incomplete combustion.
This article provides a clear and concise comparison of complete combustion and incomplete combustion. It’s a great source of information for anyone wanting to learn about these reactions.
Absolutely, Olivia55. The detailed comparison table makes it easier to understand the key differences between the two types of combustion.
The article does a great job of explaining the difference between complete combustion and incomplete combustion. It’s very well-written and educational.
I couldn’t agree more, Paul Cooper. The examples provided for complete and incomplete combustion reactions make it easier to grasp the concept.
The article offers valuable insights into the concepts of complete and incomplete combustion. It’s a great resource for anyone studying chemistry.
Absolutely, Baker Stephen. The detailed explanation of the reaction types and their consequences is very informative.
I agree, Baker Stephen. The article effectively clarifies the differences between complete and incomplete combustion, making it easier to understand.
This article sheds light on the complexities of complete and incomplete combustion reactions with great clarity. It’s a valuable resource for students and enthusiasts of chemistry.
Absolutely, Harris Charles. The detailed comparison between the two types of combustion reactions provides a deep understanding of the subject.
I couldn’t agree more, Harris Charles. The article effectively explores the science behind complete and incomplete combustion.
I found the explanation of complete and incomplete combustion reactions to be very insightful. It’s an excellent article for understanding the science behind these reactions.
I couldn’t agree more, Wlewis. The examples provided for complete and incomplete combustion reactions help in understanding the concept better.
The article is a great read for those interested in understanding combustion reactions. It effectively explains the key differences between complete and incomplete combustion.
I totally agree, Uadams. The article provides a comprehensive understanding of combustion reactions and their consequences.
The article provides a comprehensive explanation of complete and incomplete combustion along with practical examples. It greatly enhances the understanding of these reactions.
I completely agree, Rwalsh. The article effectively conveys the scientific concepts in a clear and concise manner.
The article provides an in-depth understanding of complete and incomplete combustion reactions. It’s incredibly informative and well-structured.
Absolutely, Kelly Jordan. The comparison table and examples make it easier to comprehend the complex concepts of combustion reactions.
This article clearly explains the main essence of combustion reactions in a very detailed and comprehensive manner. It’s easy to understand and very informative.
I totally agree with you, David98. The article provides a great insight into the different types of combustion reactions and their key takeaways.