Oxidation and combustion are both taught from very lower classes, and the topics are always discussed with each other. Combustion needs oxygen to fulfil the reaction.
Oxidation takes place when oxygen is added to the compound. Combustion takes place in the presence of an oxidizing agent, which can also be oxygen.
Key Takeaways
- Oxidation is a chemical reaction that involves the loss of electrons, while combustion is a type of oxidation that produces heat and light.
- Oxidation can occur without heat or light, while combustion always produces heat and light.
- Combustion requires a fuel source and an oxidizing agent, while oxidation can occur with or without a fuel source.
Oxidation vs Combustion
Oxidation is a general term that refers to any reaction in which oxygen combines with other substances to produce new compounds. Combustion specifically refers to the rapid oxidation of fuel in the presence of heat, releasing energy in the form of heat and light.

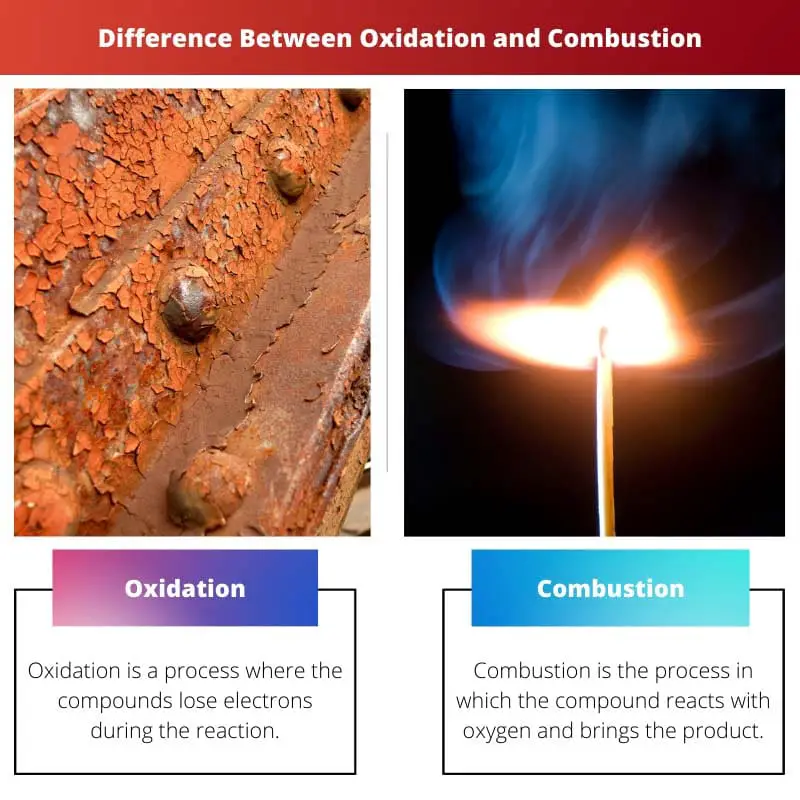
Oxidation is the process where the compound loses electrons during the reaction. This increases the oxidation state of the chemical.
Oxidation is the process in which there is no pure oxygen, but the reaction loses some electrons. The loss of electrons increases the state of the oxidation state.
Combustion is the process of burning the compounds. The compound reacts with oxygen, and the product is released in the form of heat and light. The end products do contain oxides.
Combustion ends products is an oxidation reaction sometimes. Combustion reactions can also be considered exothermic reactions as the products are released in the form of heat and light.
Comparison Table
| Parameters of Comparison | Oxidation | Combustion |
|---|---|---|
| Oxygen | Sometimes | Always |
| Electrons | Loss | Addition |
| End | Product | Light and Heat |
| Produces | End Product | Oxides |
| Other Reactions | Electrochemical reaction | Exothermic reaction |
| Relation | It does not lead to combustion. | It leads to the oxidation proces. |
What is Oxidation?
Oxidation is a process where the compounds lose electrons during the reaction. The process which undergoes oxidation has no presence or addition of oxygen in the process.
This is an issue of why the process is termed oxidation. The compounds lose one or more electrons in the process, and the loss of electrons is termed oxidation.
The oxidation state increases during the process. The electron is removed from the compounds which are part of the reaction. The oxidation process can also be termed the loss of electrons.
The opposite reaction of oxidation is reduction. When a compound is oxidized with oxygen, then it is termed the main compound oxidized.
A process undergoes oxidation, and then it also undergoes reduction. Reduction and Oxidation work hand in hand. Two half-reactions form a full reaction.
Metal displacement is a good example of the oxidation process. It shows two half-reactions and then displaced metals in the reaction. One compound will be oxidized, and the other will be reduced.
Initially, the compound was combined with oxygen, but afterwards, the meaning and the process changed. This changed the definition of the oxidation reaction.
This process was there before the invention of electrons, and after the invention of electrons, it gave a breakthrough in the process and confirmed that oxidation reaction is the transfer of electrons or loss of electrons.

What is Combustion?
Combustion is the process in which the compound reacts with oxygen and brings the product. The products are always oxides. This word means burning. The reaction can be done in high temperatures.
It releases energy during the reaction, which is released in the form of heat and light. The combustion reaction shows flames as the compounds are burned during the reaction.
Methane is the best example to see a combustion reaction. It reacts with atmospheric oxygen and produces a flame during the reaction. It produces energy which can be conveyed in electricity. The energy can also be used to cook food or to heat the water.
The products are termed oxides. Methane is a hydrocarbon, due to which the oxides are hydrogen and carbon.
The oxides are carbon dioxide and water. There are many types of combustion available. They are complete combustion or incomplete combustion and much more variety of combustion. Exothermic reactions are combustion reactions.
The flame will not create a fire as the flame just indicates the products of the reaction. The difference in the types of products the reaction leaves behind.
The oxides have a high potential and react with atmospheric oxygen or pure compounds, and emit flames. This process is also used to destroy hazardous things.

Main Differences Between Oxidation and Combustion
- Oxidation has no oxygen in the reaction, but Combustion happens in the presence of oxygen.
- Loss of electrons in oxidation, but Combustion is the addition of electrons.
- Oxidation products form from the combined compounds, but Combustion produces its products in the form of heat and light.
- Oxidation does not produce oxygen, but Combustion produces oxides.
- Electrochemical reactions are oxidation reactions, but Exothermic reactions are combustion reactions.
- Combustion produces oxidation as its end process, but Oxidation does not.
- https://www.sciencedirect.com/science/article/pii/S0043135405002538
- https://www.sciencedirect.com/science/article/pii/0167577X88900456

The content is very informative, and the examples mentioned clearly illustrate the differences between oxidation and combustion.
Absolutely, Gordon. The article’s approach to explaining oxidation and combustion is exceptional.
I concur, Gordon. The real-world examples make the article engaging and educational.
The author’s thorough examination of oxidation and combustion is truly impressive. The information shared is immensely valuable.
Absolutely, Ross. The depth of insights provided in the article is remarkable.
I couldn’t agree more, Ross. The article is an exemplary resource for understanding oxidation and combustion.
The detailed explanations about oxidation and combustion are extremely helpful. I appreciate the insights provided in this article.
The author’s ability to convey complex chemical concepts in an accessible manner is truly impressive.
I agree, Bradley. This article significantly contributes to the understanding of oxidation and combustion.
I found the article’s focus on the contrasts between oxidation and combustion to be very enlightening. The details provided are quite comprehensive.
I agree, Chelsea. The article has effectively elucidated the differences between these two fundamental processes.
The article provides valuable insights into oxidation and combustion. The comparison table and detailed descriptions are much appreciated.
I share your sentiments, Hgriffiths. This is an excellent resource for understanding chemical reactions.
Great article! I always thought oxidation and combustion were the same thing, but now I understand the differences.
I appreciate the clear explanations provided in this article.
I agree, this article is very informative and well-written.
The article offers a comprehensive comparison of oxidation and combustion, providing significant clarity on these chemical processes.
I completely agree, Kharrison. The content is well-structured and elucidates the key distinctions effectively.
It’s interesting to learn about the key differences between oxidation and combustion. The article presents a thorough comparison.
Yes, the article does an excellent job of highlighting the distinctions between oxidation and combustion.
I completely agree, Lewis. This is a great resource for understanding chemical reactions.
The information presented about oxidation and combustion is quite fascinating. The article covers the fundamental differences between the two processes effectively.
I share your sentiments, Isaac. The content is very well-researched and articulated.
I found this article to be very enlightening. The comparison table and detailed explanations really help in understanding the concepts.
I couldn’t agree more, Lee. This article provides a comprehensive exploration of oxidation and combustion.
The author’s clarity and precision in defining oxidation and combustion is commendable.