Terminologies like Elements and Atoms are frequently used while studying Chemistry and sometimes Physics. However, as the subject advances and gets more and more complicated, the meanings of the terms can get easily confused.
Key Takeaways
- Elements cannot be broken down into simpler substances by chemical means. At the same time, Atoms are the smallest particle of an element that retains the chemical properties of that element.
- Elements have unique atomic numbers that define their properties and location on the periodic table, while Atoms have protons, electrons, and neutrons that determine their behavior and interactions.
- Elements can combine to form compounds, but Atoms of the same element cannot be broken down further without changing the substance’s chemical properties.
Elements vs. Atoms
Elements and Atoms differ because a component is the most simplistic form of a substance made up of atoms. An aspect is classified depending on its atomic number. Subatomic particles combine to form atoms, which merge to create an element. Later on, elements combine to form molecules.

Unlike chemical compounds, chemical elements cannot be segmented into simpler substances by any chemical method. In the periodic table of elements, the elements are systematized by their atomic number.
An atom is the tiniest fraction of conventional matter that creates a chemical element. An atom is composed of subatomic particles. To put it in simple words, atoms are the building blocks for composing elements.
Comparison Table
| Parameters of Comparison | Elements | Atoms |
|---|---|---|
| Size | Bigger than atoms | Very small (cannot be even seen under a microscope) |
| Number of types | There are, in total, 118 elements. | There are approximately 92 kinds of atoms available in nature. |
| Composition | A specific element is comprised of only one particular type of atom. | Atoms are composed of subatomic particles. They are protons, electrons, and neutrons. |
| When they combine | Elements combine to form new chemical reactions. | When atoms combine, they form a molecule. |
| Weight | Heavier than a particular atom. | Extremely Light (Relative weight is 1 AMU) |
What are Elements?
An element is a fundamental concept one must know to understand advanced chemistry. In its atomic nuclei, an element is composed only of atoms that contain the same number of protons.
Chemical elements cannot be divided into more simplistic elements or substances using any chemical method. The latter is attributed to the atomic number, represented by the symbol ‘Z.’
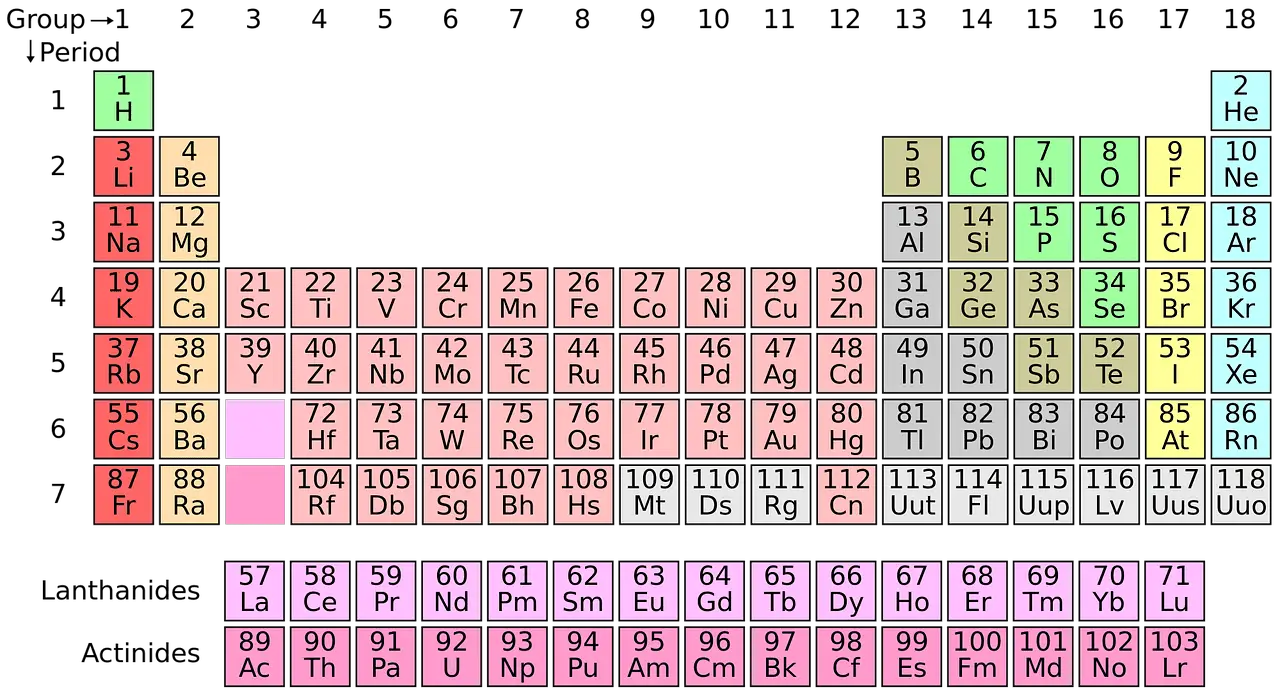
Modern chemistry depends a lot on the period table. All the discovered elements are categorized and systematically organized in the periodic table. This concept was devised by the Russian chemist Dmitri Mendeleev in 1869.
The characteristics of an element are responsible for its physical state at room or average temperature. It can be either in a gaseous state or solid or liquid.
There are approximately 118 types of elements in total.
A few examples of elements are as follows:
- Hydrogen
- Carbon
- Neon
- Magnesium
- Aluminium
- Boron
What are Atoms?
Atoms are the smallest unit of ordinary matter. They are tiny, with an approximate size of 100 picometers. Also, an atom is extremely light weighted. Relatively the weight of the latter is relatively 1AMU.
The nucleus is composed of one or more protons and several neutrons. There is only one exception to this rule: Hydrogen, which has no neutrons. The core accounts for 99.94% of an atom’s mass.
The protons possess a positive electric charge, the electrons contain a negative electric amount, and the neutrons have no electric control. An atom is electrically neutral when the number of protons and electrons are equal.
There are 92 different types of atoms in nature.
The primary concept and notion that matter comprises tiny indivisible particles are ancient. The history of atoms goes back to the ancient times of India and Greece.
Main Differences Between Elements and Atoms
- When more than one element combines, they form a new chemical reaction resulting in a new element. When more than one atom combine (keeping various matters constant), they form a molecule.
- The element’s weight is heavier when compared to that of the atom, whereas, in the case of atoms, the latter is extremely light. The relative weight of the latter is approximately 1 AMU.