The terms heterogeneous and homogeneous are mainly related to chemistry, which refers to the mixing of materials.
To know the difference between these, all we need to know is what type of ingredients are mixed, the state of the mixture formed, and whether there is uniformity in the composition or not.
To understand the different types of mixtures and their state after preparing a solution, it’s important to know the difference between heterogeneous and homogeneous, which is explained in further sections.
Key Takeaways
- Homogeneous refers to a substance with the same composition throughout, while heterogeneous refers to a substance with different compositions in different parts.
- Examples of homogeneous substances are air and water, while examples of heterogeneous substances are soil and blood.
- The substances are evenly distributed in homogeneous mixtures, while the substances are not evenly distributed in heterogeneous mixtures.
Heterogeneous vs Homogeneous
The difference between Heterogeneous and Homogeneous is that heterogeneous is a type of mixture in which the composition is not uniform, while homogeneous is a type of mixture in which the whole composition is uniform throughout the mixture, which means the component in the mixture is mixed completely with each other. The properties of both the mixture also change with the composition.

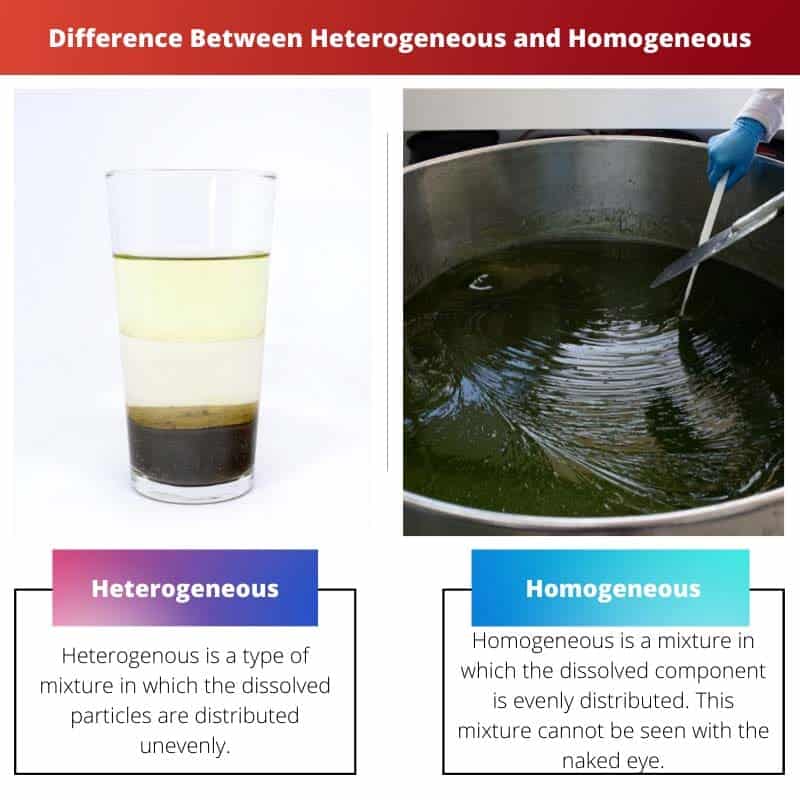
Heterogenous is a type of mixture in which the dissolved particles are distributed unevenly, and by looking at them, it can be ascertained that it is a heterogeneous mixture. Their composition is uneven.
Their particles can be easily seen in a microscope, and they have more than one phase, which can be seen with the naked eye.
A homogeneous is a mixture in which the dissolved component is evenly distributed. This mixture cannot be seen with the naked eye.
Therefore, it cannot be guessed just by looking at it. A homogeneous mixture is also called a solution.
Their composition is similar. We can only assess one phase of it.
Examples of this are vinegar, rainwater, etc.
Comparison Table
| Parameters of comparison | Heterogeneous | Homogeneous |
|---|---|---|
| Definition | Heterogenous is a type of mixture in which the dissolved particles are distributed unevenly, and by looking at them, it can be ascertained that it is a heterogeneous mixture. | A homogeneous is a mixture in which the dissolved component is evenly distributed. This mixture cannot be seen with the naked eye. |
| Uniformity | A heterogeneous is a type of mixture in which the composition is not uniform. | Homogeneous is a type of mixture in which the whole composition is uniform throughout the mixture, which means the component in the mixture is mixed completely with each other. |
| Size | The particle of the heterogeneous mixture is large. | The particle size of the homogenous mixture is small. |
| Purity | The heterogeneous mixture is not pure by nature. | The homogeneous mixture is pure by nature. |
| Types | There are two types of heterogeneous mixtures: suspension and colloids. | There is only one type of homogenous mixture and that is known as a solution. |
What is Heterogeneous?
A heterogeneous mixture is called a mixture in which the dissolved components are not equal.
For example, vegetable soup is a heterogeneous mixture because vegetables and soups can be easily separated, and all the components are present in varying amounts.
A phage is a small part of a sample with similar composition properties. A homogeneous mixture has only one phase if its definition is observed.
A heterogeneous mixture has two or more phases. If water and oil are mixed, they do not mix. They form different layers.
These layers are called phases.
It is easy to separate the components of a heterogeneous mixture. For example, if we mix sand and water to make a solution, we can see that the sand particles settle on their own after some time.
Thus we can easily separate the sand particle from the water and see the difference. Sometimes heterogeneous catalysts are also used, but the conditions are different.
The states of the reactants also differ.
Heterogeneous mixtures have variable compositions. It means that the ratio of particles is different.
Examples of heterogeneous mixtures are sand and water, a mixture of salt and iron filling, etc. there are two types of heterogeneous mixtures: suspension and colloids.

What is Homogeneous?
To define a homogeneous mixture, it is very important to know a few things, like the organized structure should be smooth and uniform.
Any substance added to a homogeneous mixture should be allowed to dissolve easily. The amount of that substance should be the same.
It is considered correct to define homogeneous mixtures as such.
There are many examples of this mixture, such as a solution of salt and water, which is the composition of a homogeneous mixture. Also, a solution of sugar and water.
Thus, it shows that both elements should not be different from each other but should be similar.
The components are almost identical in appearance and composition in a homogeneous mixture. All homogeneous mixtures are solutions.
In homogeneous mixtures, if the components are in the form of a powder and the other component is a liquid, the two mix well with each other, and those different components cannot be seen separately.
We can see this mixture only in liquid form. Air is also placed in the category of this mixture because the naked eye cannot see even its particles.
Air is a mixture of different gases which is present in our atmosphere.
Main Differences Between Heterogeneous and Homogeneous
- Heterogeneous is a type of mixture in which the composition is not uniform, while homogeneous is a type of mixture in which the whole composition is uniform throughout the mixture, which means the component in the mixture is mixed completely with each other.
- A heterogeneous mixture can easily be separated, while a homogeneous one cannot.
- The particle of the heterogeneous mixture is large, while the particle size of the homogenous mixture is small.
- The heterogeneous mixture is not pure by nature, whereas the homogeneous mixture is pure by nature.
- There are two types of heterogeneous mixture: suspension and colloids, while there is only one type of homogenous mixture, known as a solution.