There are three types of polarity and two types of covalent bonds. The three types are- polar, non-polar, and ionic. These are classified based on the force between the chemical bonds, which allows the attraction of two specific elements to each other.
The number of covalent bonds an element can form is determined by the number of vacant spaces of electrons in the element’s valance shell.
Key Takeaways
- Non-polar covalent bonds involve the sharing of electrons between atoms with similar electronegativities, resulting in a neutral charge distribution; polar covalent bonds involve an unequal sharing of electrons, creating a partial positive and partial negative charge distribution.
- Non-polar covalent bonds occur between atoms of the same element or between atoms with similar electronegativities, such as C-H bonds; polar covalent bonds occur between atoms with different electronegativities, such as O-H or N-H bonds.
- Non-polar molecules have no net dipole moment; polar molecules have a dipole moment due to the uneven charge distribution.
Non-Polar vs. Polar Covalent Bonds
Elements with different electronegativity form polar covalent bonds. Bonds are formed with the transfer of electrons among elements. Polar covalent bonds can conduct electricity and have high melting and boiling points. Elements with the same electronegativity forms non-polar covalent bonds. Non-polar covalent bonds cannot conduct electricity and have low melting and boiling points.

The non-polar and polar covalent bonds fall under the classification of covalent bonds. They occur in non-metal and two different types of elements.
This classification also tells the sharing and distribution of electrons in the two elements and the resulting electronegativity between them. The formation of bonds occurs when the elements combine, and some of the electrons from one element transfer to another.
This transfer can either result in equal or unequal sharing of electrons. The electronegativity difference between them determines the type of bond that will form between these elements.
Comparison Table
| Parameters of Comparison | Non-polar Covalent Bonds | Polar Covalent Bonds |
|---|---|---|
| Definition | Non-polar covalent bonds are bonds between elements that have the same electronegativity. | Polar covalent bonds are bonds between elements that have different electronegativity. |
| Electron cloud | The electron clouds in these molecules are not distorted. | The electron cloud in these molecules is distorted. |
| Charge build-up | There is no charge build-up in these elements. | There is a charge build-up at the poles of these elements. |
| Dipole bond | Non-polar covalent bonds have no dipole moment. | Polar covalent bonds have a dipole moment. |
| Force between molecules | There are weak Van der Waal forces of attraction between the molecules. | There are stronger forces of attraction than Van der Waal’s forces between the molecules. |
| Melting and boiling points | These compounds have lower melting and boiling points than polar covalent bond molecules. | These compounds have higher melting and boiling points. |
| Conducting electricity | These compounds do not conduct electricity. | These compounds conduct electricity in aqueous solution. |
What is Non-Polar Covalent bond?
When electrons are shared equally between two atoms, a chemical bond is formed, called a non-polar covalent bond. This is why the electrons shared by each atom in these molecules are the same.
Also, the electronegativity between these atoms is almost negligible. In other words, both atoms have similar electronegativity and no charge separation.
This type of bond can also be formed when the atoms sharing a polar bond arrange so that the electric charges between them cancel out each other. These types of bonds occur between different or identical atoms that are non-metals.

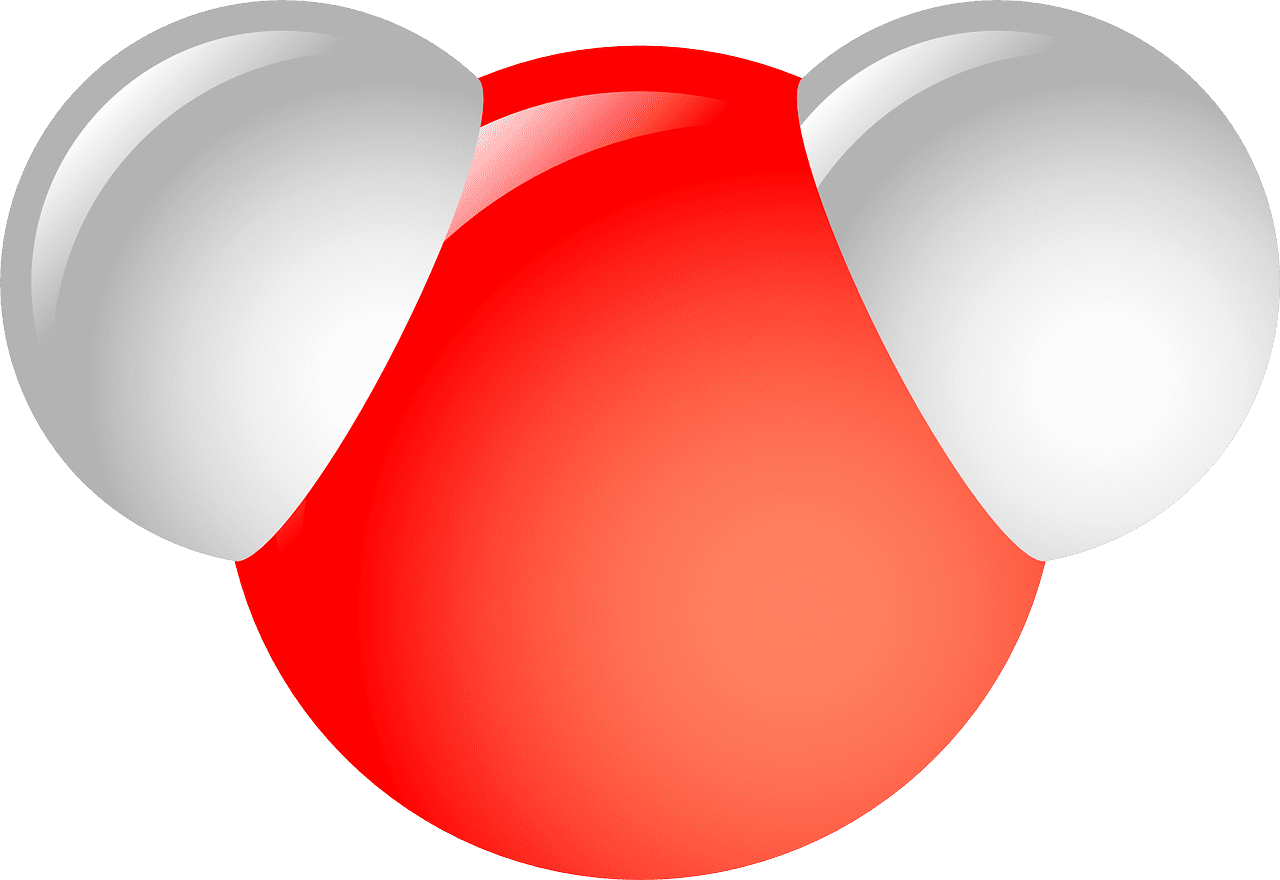
What is a Polar Covalent bond?
The bond between two atoms whose electrons are not evenly distributed is called a polar covalent bond. Polar covalent bonds can be a dividing line between the formation of an ionic bond and a pure covalent bond.
Due to this, there is always an electric dipole moment in these molecules where the two ends are relatively negative or positive. They are formed between two non-metal atoms that have different electronegativity.
The compounds with this bond can exist as solids due to a greater force of interactions. Also, the melting and boiling points of these compounds are very high.
They can conduct electricity if dissolved in an aqueous solution. These compounds are easily soluble in polar solvents like water.
Main Differences Between Non-Polar and Polar Covalent Bonds
- Non-polar covalent bonds are bonds between elements with the same electronegativity, whereas polar covalent bonds are between elements with different electronegativity.
- Non-polar covalent bonds have weak Van der Waal’s forces of attraction, while polar covalent bonds have stronger forces of attraction than Van der Waal’s forces between them.
- The electron clouds in non-polar covalent bond molecules are not distorted, whereas those in polar covalent bond molecules are distorted.
- Non-polar covalent bond compounds have lower melting and boiling points than polar covalent bond molecules.
- Non-polar covalent bond compounds do not conduct electricity, whereas polar covalent bond molecules can conduct electricity in aqueous solutions.
- Non-polar covalent bond molecules have no dipole moment, whereas polar covalent bond molecules have a dipole moment.
- There is no charge build-up in non-polar covalent bond molecules, while there is a charge build-up at poles in polar covalent bond molecules.




