Nucleic acids in biology are meant chemical compounds that occur naturally and serves as the key information-carrying particle in cells. Nucleic acids, including RNA and DNA, are monomeric nucleotide polymers.
Each nucleoside is mainly made up of sugar (5-carbon), a nitrogenous base (or nucleobase), and phosphoric acid. Five nucleobases mainly serve as the fundamental units of the genetic code.
The names of those nucleobases are uracil, thymine, cytosine, guanine, and adenine. In this article, the adenine and guanine differences are highlighted.
Key Takeaways
- Adenine and Guanine are both nitrogenous bases found in DNA and RNA.
- Adenine pairs with thymine in DNA and uracil in RNA, while Guanine pairs with cytosine.
- Adenine has a single-ring structure called a purine, while Guanine has a double-ring structure.
Adenine vs Guanine
Adenine contains an amine group on C-6 while guanine’s amine group is on C-2. Adenine in its pyrimidine ring contains an added double bond between N-1 and C-6 while guanine has a carbonyl group on C-6 in its pyrimidine ring. Adenine is soluble in water while guanine is insoluble in water.
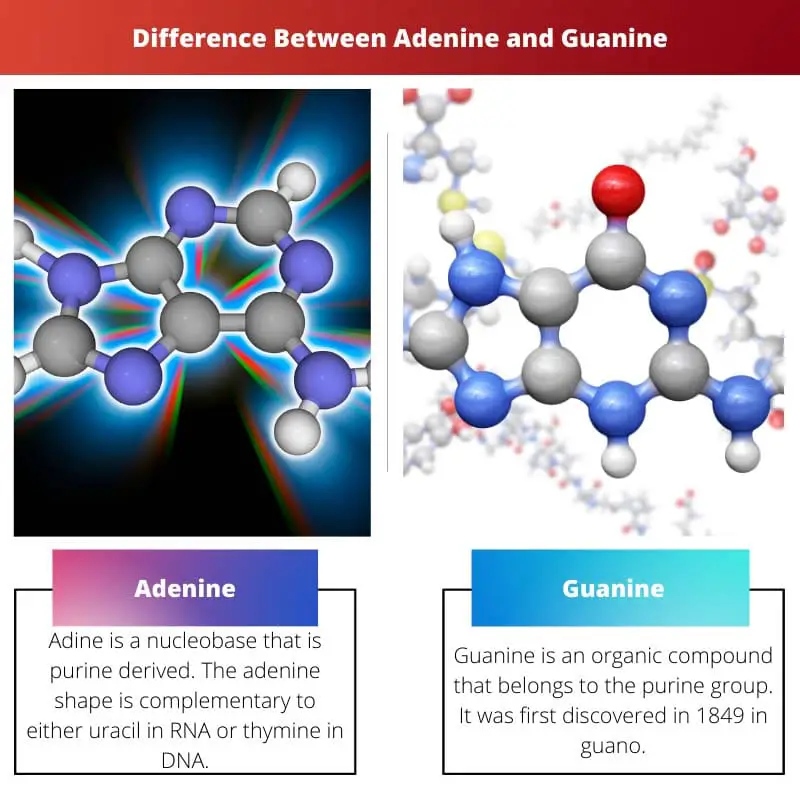
Adine is a nucleobase that is purine derived. The adenine shape is complementary to either uracil in RNA or thymine in DNA.
It has a chemical component of RNA and DNA functions in protein synthesis. Biochemistry has a variety of roles, including cellular respiration.
Guanine is an organic compound that belongs to the purine group. It was first discovered in 1849 in guano.
In 1891, it was isolated from nucleic acids. Deoxyguanosine and nucleosides guanosine are more complex compounds obtained from nucleic acid.
GTP or guanosine triphosphate is the guanylic acid unit in RNA in the body.
Comparison Table
| Parameters of Comparison | Adenine | Guanine |
|---|---|---|
| Chemical formula | C5H5N5 | C5H5N5O |
| Molecular mass | 135.13 g/mol | 151.13 g/mol |
| Solubility in water | 0.103 g/100 mL | Insoluble in water |
| Density | 1.6 g/cm3 | 2.200 g/cm3 |
| Melting point | 360 to 365 °C | 360 °C |
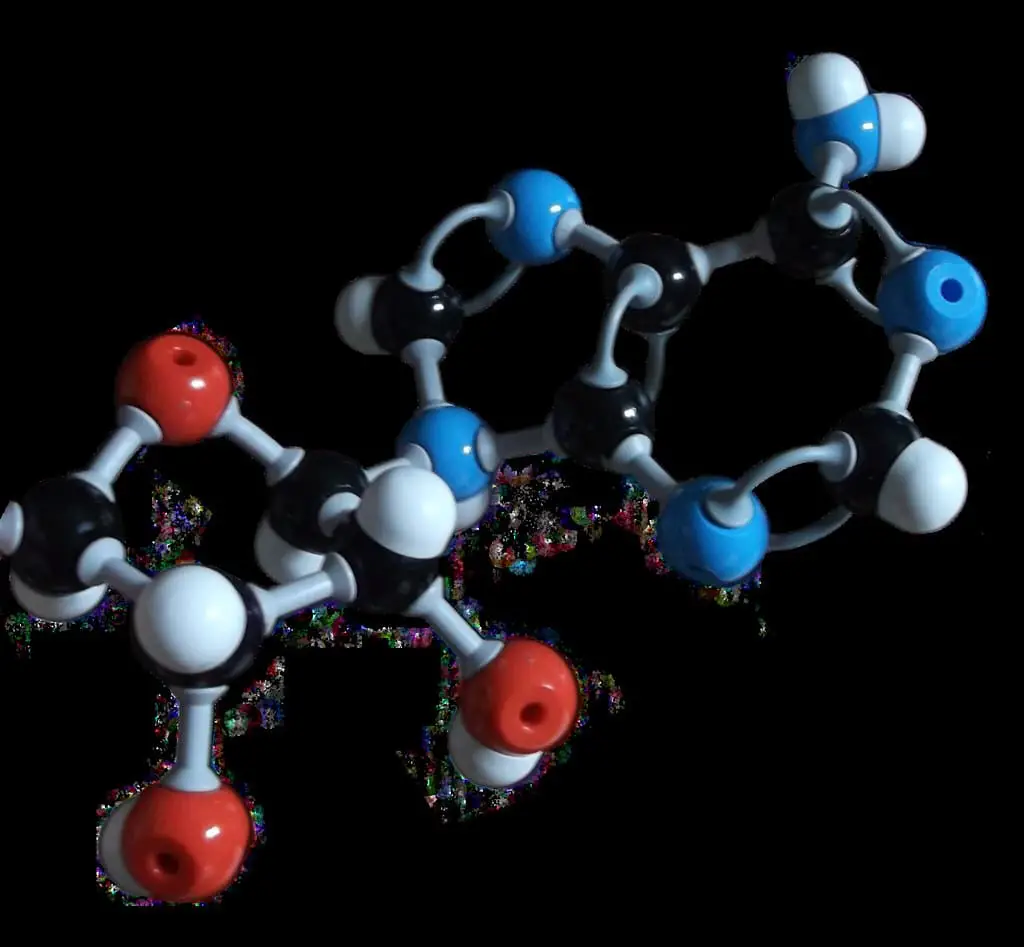
What is Adenine?
Adenine is a nucleobase in purine form. Purines are organic compounds derived from heterocyclic aromatic.
It mainly consists of two carbon rings, imidazole and a pyrimidine ring. When adenine is a constituent of DNA, with the help of a covalent bond, it is linked to deoxyribose sugar and is called an adenine residue.
Purine metabolism (including adenine) has the metabolic end product known as uric acid. The kidney, liver, and other internal organs have high purine.
They are also present but in moderate amounts in seafood, beans, meat, mushrooms, and cauliflower. As it is clear that adenine is a vital component of nucleic acids, but it is also a major constituent of ATP. (Adenosine triphosphate).
In which adenosine is attached with three phosphate groups. ATP is a molecule with energy-rich and is mainly used in biological reactions such as cellular metabolism.
Adenine forms many tautomers they are compounds that are considered equivalent and can be rapidly interconverted. But in isolated conditions, such as in an inert gas matrix, tautomer is mainly found in 9H-adenine.
When it comes to appearance, adenine is crystalline and ranges from white to light yellow.
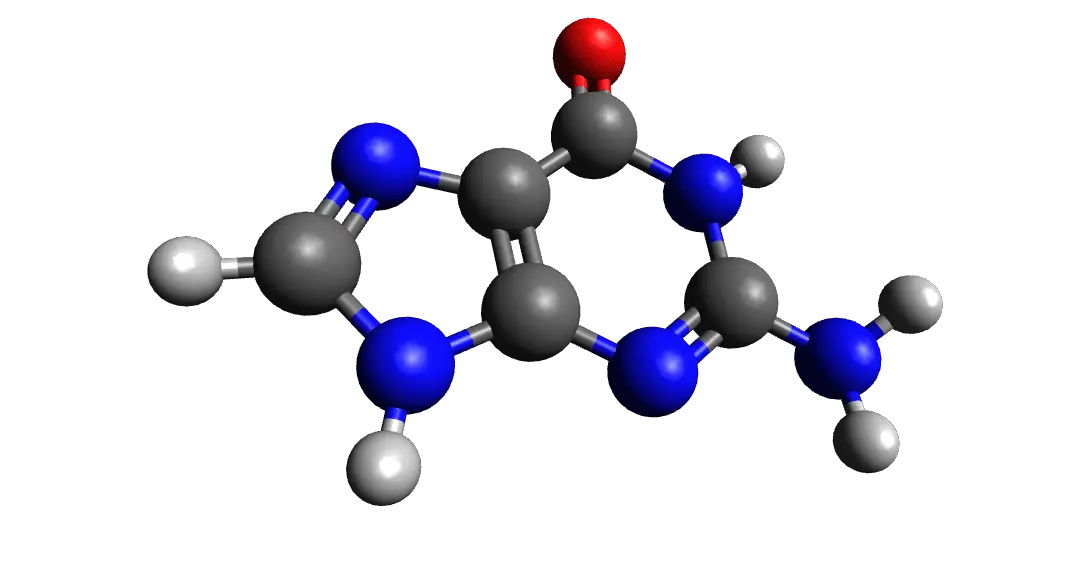
What is Guanine?
Among the main nucleobases, guanine is one of them. When it comes to pairing, guanine is paired in DNA with cytosine.
Guanosine is referred to as the guanine nucleoside. Guanine is also a derivate of purine and comprises a ring system of a fused pyrimidine-imidazole along with conjugated double bonds.
Guanine is available in both DNA and RNA, while uracil is present only in RNA, and thymine exists in DNA. The rare enol form and the major keto form are two of the tautomeric forms of guanine.
Strong acids such as carbon monoxide, ammonia, glycine, and carbon dioxide can be easily hydrolyzed in guanine. It has the carbonyl group of C-6, which acts as a hydrogen bond acceptor.
On the other hand, the C-2 group of amino and the N-1 group acts as the donors of the hydrogen bond. It is not synthesized de novo, alternatively, with the help of an enzyme, namely guanosine phosphorylase is split from the guanosine, more complex guanosine.
To turn into xanthine, guanine gets deaminated. Guanine oxidizes more readily compared to adenine.
The high melting point of guanine at 360 °C reflects the crystal’s molecules’ bonding of hydrogen between the amino groups and the oxo. Guanine manages intracellular signalling networks for communication within the cell.
Main Differences Between Adenine and Guanine
- In terms of complementary base pair, adenine forms a pair with uracil in RNA and thymine in DNA, whereas guanine forms a pair with cystonine in both RNA and DNA.
- When it comes to appearance, adenine is crystalline and ranges from white to light yellow. But guanine has a white amorphous solid appearance.
- The functional group of adenines in the amine group on the bond of C-6 in its ring of pyrimidine, whereas guanine consists of C-6 in the carbonyl group and C-2 in the amine group in its ring of pyrimidine.
- In adenine, FAD, NAD, and ATP serve as energy carriers. On the flip side, GTP in guanine serves as a second messenger.
- Adenine forms energy by binding with other nucleotides, which is vital for cellular function, while guanine manages intracellular signalling networks for communication within the cell.