Ketones and aldehydes are two types of organic molecules. These can be produced artificially, however, there are several natural substances. It’s possible that the two were confused because of their chemical components.
Both have a carbonyl group and are organic compounds. The carbon atom in this group possesses a double hydroxyl bond. This indicates that a trigonal planar configuration in ketones and aldehydes surrounds the carbonyl carbon atom.
Nevertheless, there are substantial distinctions between the two functional categories, and the purpose of this essay is to assist you in distinguishing between them.
Key Takeaways
- Aldehydes contain a carbonyl group bonded to a hydrogen atom, whereas ketones have a carbonyl group bonded to two carbon atoms.
- Aldehydes show higher reactivity than ketones due to the presence of a hydrogen atom, making them more susceptible to nucleophilic attack.
- Aldehydes undergo oxidation more easily than ketones, forming carboxylic acids and alcohols, respectively.
Aldehydes vs Ketones
Aldehydes are known as organic compounds or acids composed of a double carbon and oxygen bond with two aryl groups or alkyl groups around the two bonds of carbonyl carbon atom. Ketones are organic compounds with a double bond of carbon and oxygen (C=O). The dipole of carbonyl carbon has two methyl groups attached to it in ketones.

An aldehyde is a molecular compound containing a functional group of the formula CHO, consisting of a carbonyl centre linked to an H and any basic alkyl or chain length R group. An aldehyde or formyl group is the functional group altogether.
A ketone (/kiton/) is a compound that contains biochemistry having the formula R2C=O, wherein R can be any carbon-containing ligand. Carbonyl groups are found in ketone molecules.
Acetone (R = R’ = dimethyl) is from the most basic ketones, with the formula CH3C(O)CH3. Ketones have an important role in biological chemistry as well as in the industry.
Examples are numerous sugars (ketoses), many hormones (e.g. testosterone), and the solvents acetone.
Comparison Table
| Parameters of Comparison | Aldehydes | Ketones |
|---|---|---|
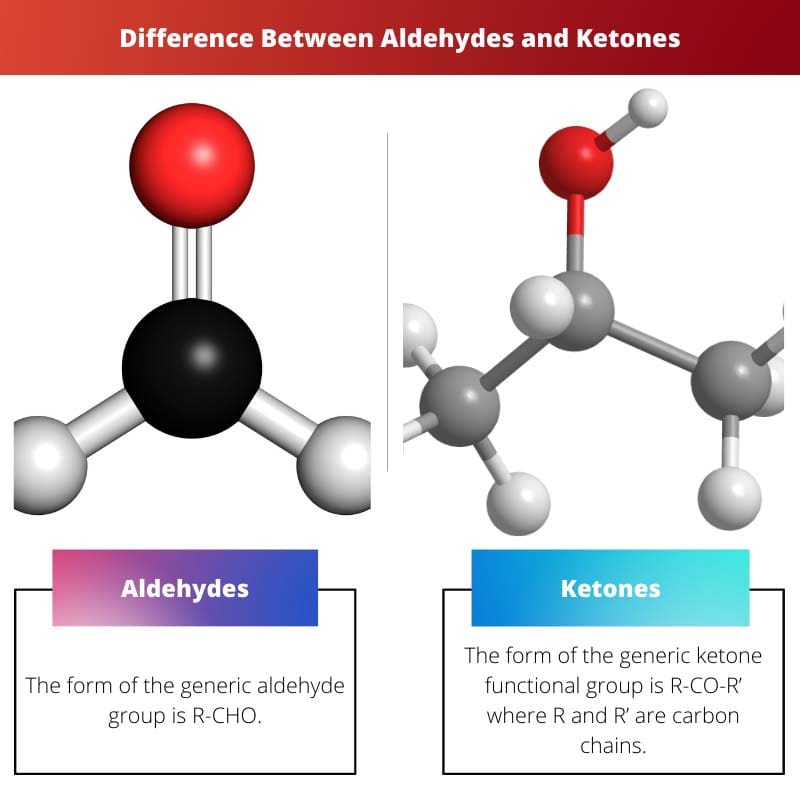
| Structure | The form of the generic aldehyde group is R-CHO. | The form of the generic ketone functional group is R-CO-R’ where R and R’ are carbon chains. |
| Definition | An aldehyde is a molecular compound that contains a functional group of the formula CHO. | A ketone is a compound that contains in biochemistry having the formula R2C=O, wherein R can be any carbon-containing ligand |
| Reactivity Magnitude | Very reactive | Less reactive |
| Occurrence | Terminal of a carbon chain. | Always occurs in the middle of a carbon chain. |
| Fehling’s Test | Pink-red precipitate is formed. | No reaction |
| Tollen’s Test | Black precipitate is obtained. | None |
| Sodium Hydroxide Test | Brown gel-like precipitate. | None |
| Schiff’s Test Results | Pink color. | No color. |
What is Aldehyde?
The aldehyde is an important biochemical and industrial functional group that consists of a carbon atom connected to a hydrogen atom and then double-bonded to an oxygen atom (chemical formula O=CH-).
Alcohol that has been dehydrogenated appears to be the source of the term aldehyde. Aldehydes were once termed by the alcohols they were related to, such as vinous aldehyde for acetaldehyde.
(Vinous comes from the Latin vinum, which means wine, which contributes to the onset of ethanol) The aldehyde family is polar. Because oxygen is less electropositive than carbon, it attracts the electrons inside the carbon-oxygen bond.
It joins with a hydrogen atom on the opposite end. Formaldehyde is considered the most basic kind of aldehyde.
The fact that formaldehyde deviates from the conventional equation by possessing a hydrogen atom rather than the R group is what makes it so popular.
The carbonyl group is connected to two H atoms in formaldehyde, the most fundamental aldehyde. The carbocation is linked to one hydrogen but another carbon group in many other aldehydes.
The carbocation of an aldehyde is expressed as CHO in the dimension of sustainability formulae. The equation for formaldehyde is HCHO, while the equation for acetaldehyde is CH3CHO.
What is Ketone?
The term ketone comes from the ancient German word aketon, which means “acetone.” Ketone designations are created by altering the original alkane’s suffix -ane to -anyone, as per IUPAC nomenclature guidelines.
Although the location of the nucleophile carbonyl functional group is indicated by a number, traditionally non – measurable names for the most prominent ketones, such as acetone as well as benzophenone, are still widely used.
Ketone C-H bonds close to the carbonyl are much more acidic (pKa 20) compared to alkane C-H interactions (pKa 50). This discrepancy is due to the electrophile ion’s resonance stability after dissociation.
The relative ph of the- hydrogen is crucial in the enolization processes of ketones and other oxidation products.
Because of the -hydrogen’s acidity, ketone and other oxidation products can react as catalysts with molar ratio and catalytic bases at that location.
The substituents in ketones are used to classify them. The equivalence of the two organic methyl groups connected to the carbonyl core differentiates ketones into symmetrical and asymmetric derivatives, according to one general categorization.
Acetone (C6H5C(O)C6H5) is a symmetric ketone, as is benzophenone (C6H5C(O)C6H5). Acetophenone is a ketone with an asymmetric structure.
Main Differences Between Aldehydes and Ketone
- Aldehydes are a more reactive functional group than the ketone group.
- Aldehydes are situated at the terminal or end of a carbon chain, whereas a ketone group occurs in the middle of a carbon chain.
- In Schiff’s test, aldehydes give out pink, whereas ketones give out no color.
- Aldehydes give out black-coloured precipitate, whereas ketones give out no reaction to this test.
- The structure of aldehyde is R-CHO, whereas the structure of ketone is R-CO-R’.