Actions and reactions always go hand in hand. The environment is subject to various types of reactions depending upon their reasons.
Chemical and Physical elements of the environment go through certain changes which result in reactions, chemical or physical, as termed in science.
These reactions are nothing but different kinds of environmental changes that either occur naturally or artificially by introducing a separate substance into the element.
Key Takeaways
- Chemical reactions result in the formation of new substances.
- Physical reactions do not lead to the formation of new substances but only affect the state of matter.
- Chemical reactions involve the release or absorption of energy.
Chemical vs Physical Reaction
The difference between Chemical and Physical Reactions is that a Chemical reaction results in the formation of a completely new substance, whereas a Physical reaction results in the reorganisation of the original molecule. A chemical reaction is irreversible, while a physical one is easily reversible. When the physical reaction is a temporary change, the chemical reaction is a permanent one.

A chemical reaction is a process in which the atoms of an element go through changes. As a result, rearrangement of the atoms takes place, or a completely new substance gets formed.
The chemical properties and configuration of a substance change when it undergoes a chemical reaction. The ones that go through the reaction are known as reactants, and the resulting substance is known as the product.
On the other hand, a physical reaction is a process in which an element changes its physical characteristics, such as state, shape, size, appearance etc.
In a physical reaction, no new substance is formed. The original substance just has some rearranged physical properties, but its molecular composition remains the same.
Comparison Table
| Parameters of Comparison | Chemical Reaction | Physical Reaction |
|---|---|---|
| Meaning | When the chemical composition of an element changes, it is called a chemical reaction. | When the physical properties of an element change but molecular composition remains the same, it is called physical reaction. |
| Reversibility | It is irreversible. | It is easily reversible. |
| New Substance | A new substance gets formed. | No new substance is formed. |
| Type | It is a permanent change. | It is a temporary change. |
| Enery Generation | Energy is generated in the form of heat, sound or light. | No energy generation occurs. |
| Energy Absorption | Energy absorption occurs. | Little to no energy absorption occurs. |
| Examples | Burning of candle, digestion of food, rusting etc. | Boiling, freezing and melting of water etc. |
What is Chemical Reaction?
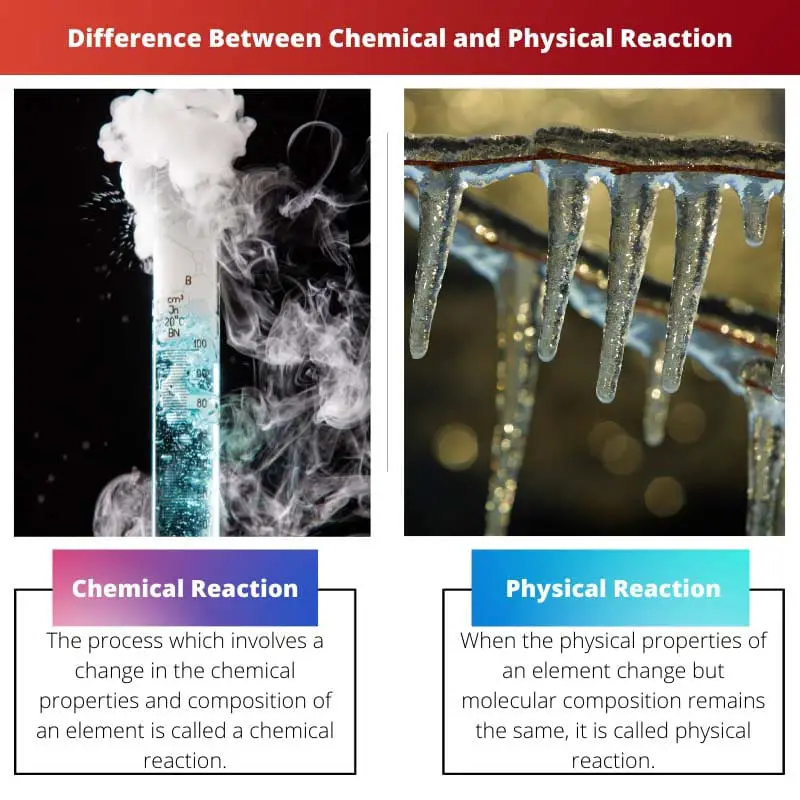
The process which involves a change in the chemical properties and composition of an element is called a chemical reaction.
The substance or reactants go through a reaction, which changes their chemical characteristics and forms a new substance or product. The change is permanent and cannot be altered.
A chemical reaction is irreversible, i.e. it cannot be easily brought back to its original form. Once the chemical composition changes, it will require another alteration (if possible) to convert into its original form.
The change occurs when bonds between certain molecules are made or broken. An element with a particular set of chemical properties is either altered or fused with another element for the formation of the new element.
After a chemical reaction, various types of changes can be seen in the resulting substance. There is a change in the temperature, i.e. either it increases or decreases.
A colour shift might be observed due to alteration of the original colour or by mixing two colours. A change in its taste is also possible. After the reaction, a solid material can be observed at the end of the container called a precipitate.
Chemical reactions can be of different ranges and types. The burning of a candle, burning of natural gas in a furnace, electrolysis, precipitation, and fermentation of grapes are all examples of it.

What is Physical Reaction?
The process which involves a change in the physical properties of an element is called a physical reaction.
A substance goes through a reaction that changes its physical characteristics, but its chemical composition remains the same. The change is temporary and can be altered easily.
A physical reaction is easily reversible, i.e. it can be brought back to its original form once again. The changes are not very stable and can be converted by basic physical methods.
An element having a certain shape, size, volume etc., experiences a change either naturally or using physical procedures.
After a physical reaction takes place, changes in the physical properties of the substance undergoing the reaction can be observed. Shape, size, colour, volume, physical appearance, and state of the substance get changed.
However, the molecular composition remains similar to the initial state. No new substance is formed; only alterations occur instead.
Just like chemical reactions, physical reactions can also possibly be of various ranges. Boiling water, melting wax, chopping wood, and crumpling paper are all examples of it.

Main Differences Between Chemical and Physical Reaction
- The chemical reaction changes the chemical composition, while the Physical reaction changes the physical properties of an element.
- A chemical reaction is irreversible, but a physical reaction is easily reversible.
- Energy generation takes place in the case of a chemical reaction alone.
- A chemical reaction is a permanent alternation, but a physical reaction is just a temporary one.
- A new substance is formed only in a chemical reaction.
- Energy absorption occurs at a high rate in a chemical reaction, while in a physical reaction, there is just little or no energy absorption.

The comprehensive explanation of chemical and physical reactions in this article is very informative. It’s helpful to have a clear understanding of these concepts.
Absolutely, the clarity of information presented here is beneficial for anyone interested in environmental science and chemistry.
The explanations about chemical and physical reactions are very well articulated. The article effectively illustrates the fundamental concepts involved in these reactions and their impact on the environment.
Indeed, these scientific explanations help enhance our understanding of the natural world and how various reactions shape it.
The differentiation between chemical and physical reactions is enlightening. It’s intriguing to learn about the distinct characteristics and implications of these reactions on different environmental elements.
Absolutely, understanding these reactions is crucial for comprehending the transformations in our environment.
I completely agree. This article provides valuable insights into the science of reactions and their effects on the surroundings.
Thank you for such an in-depth explanation of chemical and physical reactions. It’s interesting to understand the differences and similarities between the two types of reactions.
I totally agree. This post is very informative, and it’s great to learn more about this topic.
This article effectively highlights the key differences between chemical and physical reactions, providing a thorough understanding of the environmental changes resulting from these reactions.
Agreed; the detailed comparisons and examples contribute significantly to understanding the principles discussed.
Absolutely, the detailed information presented here is valuable for those seeking knowledge about reactions and their effects.
The detailed comparison between chemical and physical reactions is incredibly informative. It provides a solid understanding of the fundamental differences between these reactions and their impact on the environment.
I appreciate the detailed comparison table provided in this article, which succinctly outlines the differences between chemical and physical reactions. It’s an excellent reference for future use.
I couldn’t agree more. Having a comprehensive comparison makes it easier to grasp the distinctions.
This article provides a clear distinction between chemical and physical reactions, shedding light on how they differ in terms of reversibility, generation of new substances, and energy changes.
Absolutely, this is valuable information for anyone interested in science and environmental studies.
Yes, it’s essential to understand these differences to recognize and interpret diverse reactions in our environment.
This article offers a valuable comparison table, making it easier to grasp the distinctions between chemical and physical reactions. The detailed insights enhance clarity in understanding these concepts.
Exactly, having a comparative overview allows for a better understanding of the complexities related to these reactions.
Absolutely, the clarity of information presented here is beneficial for anyone seeking knowledge about reactions and their impact on the environment.
The detailed explanations about chemical and physical reactions contribute to a comprehensive understanding of these concepts. The examples provided further enhance the clarity of the content.
I completely agree. The practical examples make it much easier to relate to the scientific principles presented.
Indeed, having detailed examples helps in illustrating the practical implications of chemical and physical reactions.