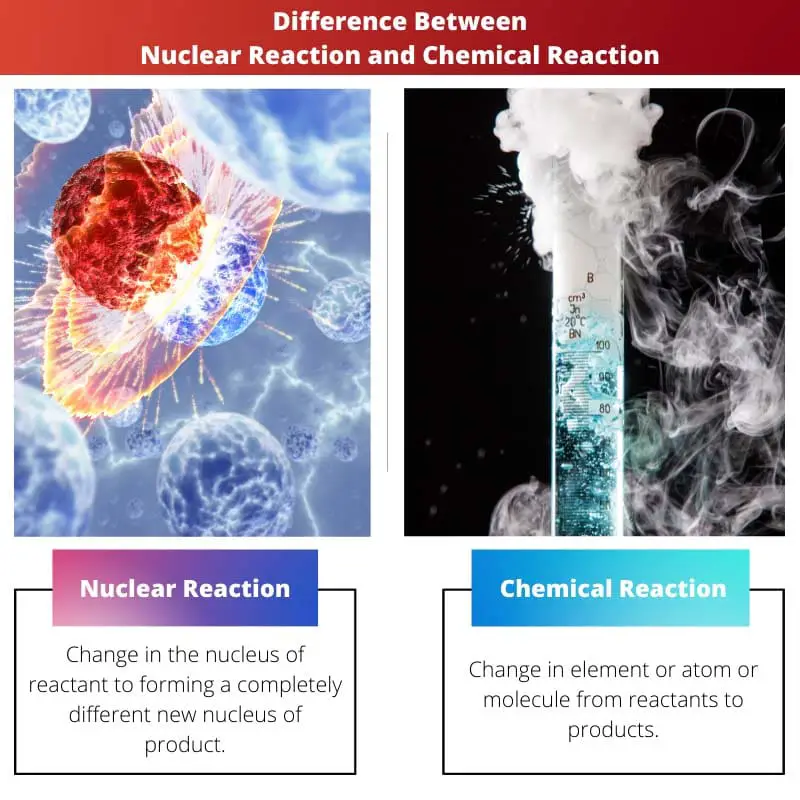
In nature, numerous reactions are going down every minute or second. The smallest of things undergo different types of reactions.
The food the plant produces, the digestion, any chemical production, changing of the shapes, etc., are some reactions that are commonly seen.
Also, in laboratories, one can study these reactions at a very close glance. The production of dissociation of any atoms, molecules, nucleus, etc., is also classified as one of them.
Key Takeaways
- Nuclear reactions involve changes in the nucleus of an atom, while chemical reactions involve changes in the electron configuration of an atom.
- Nuclear reactions release a huge amount of energy, while chemical reactions release a relatively small amount.
- Nuclear reactions can result in the formation of new elements, while chemical reactions do not.
Nuclear Reaction vs Chemical Reaction
A nuclear reaction is a reaction in which one or more nuclei collide to produce new nuclei. This type of reaction releases high amounts of heat. A chemical reaction is the type of reaction in which atoms are rearranged to form new arrangements of an element. It releases low amounts of heat.
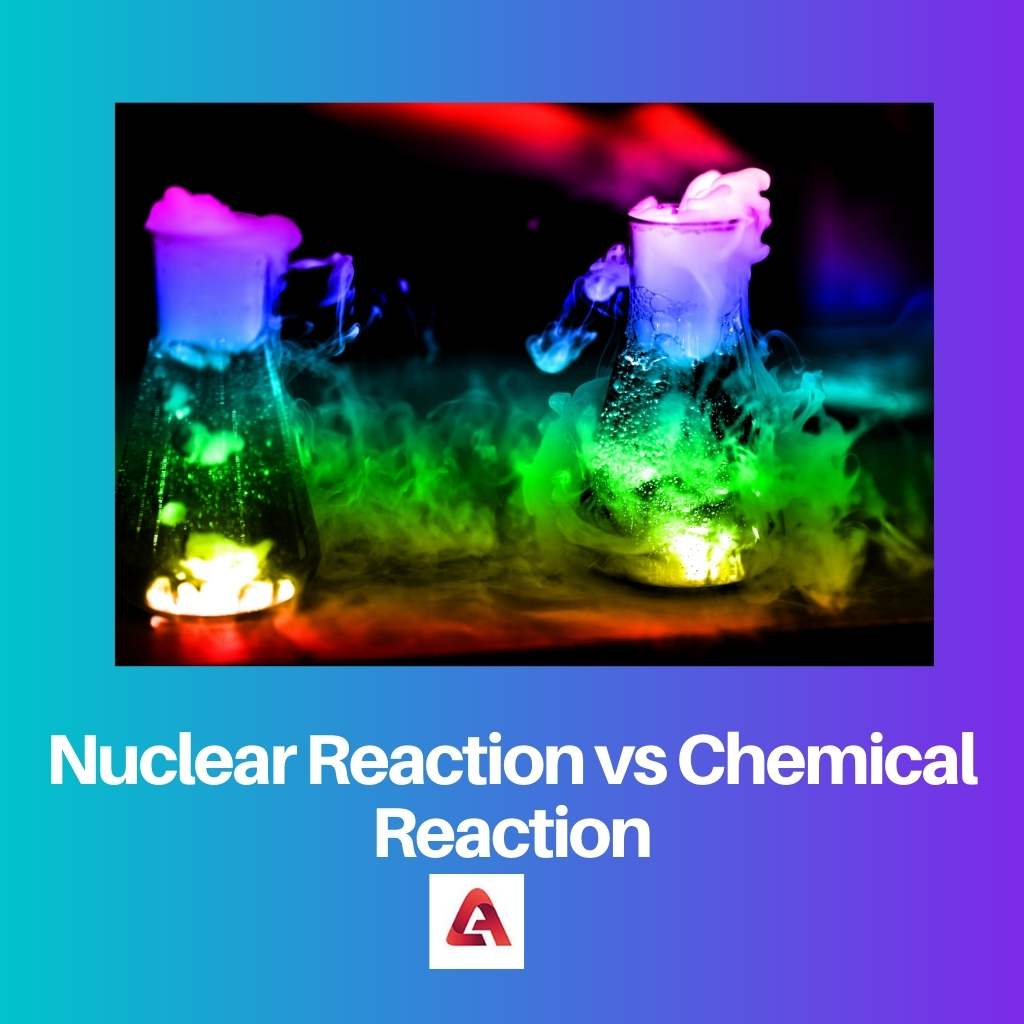
A nuclear reaction is a type of reaction that produces an enormous amount of heat while taking place. Also, the amount of heat produced is from the destruction of mass.
The reaction, which is considered to be irreversible in most cases, does not conserve mass.
The interesting fact about the reaction is that they are nearly independent of external factors such as pressure, temperature, etc.
A chemical reaction is a type of reaction that is carried out mostly in laboratories safely. While in a chemical reaction, heat is released but not in a huge range like that of a nuclear reaction.
The chemical reactions are somewhat dependent on the external factors that may bring changes in the products, and that may include – pressure, temperature, the activity of the catalyst, etc.
Comparison Table
| Parameters of Comparison | Nuclear Reaction | Chemical Reaction |
|---|---|---|
| Place of Occurrence | Inside the nucleus of the atom or element or molecule | Outside the nucleus of the atom or element or molecule |
| What is it? | Change in the nucleus of reactant to forming a completely different new nucleus of product | Change in element or atom or molecule from reactants to products |
| External Factors | Independent in nature | Dependent in nature |
| Energy Release | An enormous amount of energy | A low range of energy is released. |
| Bond Formation | No such activity takes place | New bonds are formed |
| Nature | Irreversible | It can be both reversible or irreversible |
| Example | Beta-decay of 14C | Formation of NaCl |
| Units | Million electron volts/individual nucleus | KJ/mol or J/mol |
What is Nuclear Reaction?
Nuclear reactions are reactions that can be defined or stated as the reactions that occur when one or two nuclei of an atom, element, or molecule collide with each other to form new nuclei of an atom, element, or molecule.
During the ongoing nuclear reaction, scientists have found that they release a tremendous amount of energy that can be stored as nuclear energy in reactors, termed nuclear power.
The advantage of nuclear reaction is that even a small amount can produce a large sum of energy. These are of two types – nuclear fission and nuclear fusion.
Among the two types of reaction, nuclear fission is the reaction under controllable by humans in which the energy is uncontrollable, whereas nuclear fusion is the reaction that takes place in the sun, which is the ultimate source of energy.
What is Chemical Reaction?
A chemical reaction is a reaction that can be defined or stated as the reaction that takes place when there is a rearrangement of the atoms or elements or molecules of reactants in something different to form a new product with a different arrangement of atoms or molecules or elements.
While in an ongoing chemical reaction, the amount of heat released is low and is expressed in terms of J/mol or KJ/mol.
The chemical reaction doesn’t involve the nucleus while it occurs.
Also, these reactions are hugely dependent on the external factors that are responsible for making a change in them, and they are – pressure, temperature, pH, rate of reaction, the activity of the catalyst, etc.
Thus, they may be reversible or irreversible, depending on their type.

Main Differences Between Nuclear Reaction and Chemical Reaction
- Nuclear reactions are a reaction that occurs inside the nucleus of any element atom or molecule, whereas comparatively, on the other hand, a chemical reaction is a reaction that occurs outside the nucleus of the element atom or molecule.
- A nuclear reaction, in general, can be defined or stated as the reaction that occurs inside the nucleus and changes the nucleus of one element atom or molecule to another one to form a completely different one, whereas comparatively, on the other hand, the chemical reaction, in general, can be stated or defined as the reactions that take place to change element or atom or molecule from one to another without any change in the nucleus.
- Nuclear reactions are said to be independent of the external factors that affect any reaction, which may include – pressure, temperature, pH, rate of reaction, and many more others, on the other hand, a chemical reaction is a reaction that is not independent to external factors, despite the fact that they are the one that is mostly affected by the difference in pressure, temperature, pH, rate of reaction, catalyst activity, and many more others.
- Performing a nuclear reaction it is believed to be releasing an enormous amount of heat, whereas comparatively, on the other hand, the amount of heat released while performing any chemical reaction is lower than that of a nuclear reaction.
- In a nuclear reaction, there is no breaking of bond while forming any new element or atom or molecule, while comparatively, on the other hand, in a chemical reaction, there is the formation and breaking of a bond to form a new element or atom or molecule.
- The nuclear reactions are mostly irreversible, while comparatively, on the other hand, the chemical reaction might be reversible or irreversible.
- The energy released in a nuclear reaction is expressed in terms of million electron volts/individual nucleus, while comparatively, on the other hand, the energy released in a chemical reaction is expressed in terms of J/mol or KJ/mol.
- The beta decay of 14C is an example of a nuclear reaction, while comparatively, on the other hand, the formation of NaCl is a common example of a chemical reaction.