Nutrients in food cannot be absorbed by the body straight away. They need to be broken down into smaller parts by various enzymes.
In the case of proteins, specific enzymes aid in breaking them into amino acids that the body uses. These enzymes are of two types, namely chymotrypsin, and trypsin.
Key Takeaways
- Chymotrypsin is a proteolytic enzyme that cleaves peptide bonds at aromatic amino acids.
- Trypsin is another proteolytic enzyme that targets peptide bonds at basic amino acids.
- Both enzymes play essential roles in protein digestion within the digestive system.
Chymotrypsin vs Trypsin
Chymotrypsin is a digestive enzyme that breaks down proteins into smaller peptides and amino acids. Trypsin cleaves peptide bonds on the carboxyl side of essential amino acids. Trypsin is produced in the pancreas and is important for the digestion of proteins in the small intestine.

Chymotrypsin is an enzyme that essentially aids the digestion process by breaking down proteins. It is secreted from the pancreas as a constituent of pancreatic juice.
The enzyme is activated by its precursor, which is called chymotrypsinogen. This is an inactive enzyme that only functions in the presence of Trypsin.
Meanwhile, trypsin is another kind of digestive enzyme that works with different amino acids. It is produced by the pancreas as well.
However, most of its work is carried out in the small intestine. Its precursor is an inactive enzyme called trypsinogen. The inactive enzyme functions only in the presence of enterokinase.
Comparison Table
| Parameters of Comparison | Chymotrypsin | Trypsin |
|---|---|---|
| Discovery | It was discovered in the 1900s. | It was discovered in 1876. |
| Meaning | It is a digestive enzyme that works on breaking down aromatic amino acids. | It is a digestive enzyme that works on breaking down basic amino acids. |
| Precursor | Its precursor is an inactive enzyme called chymotrypsinogen. | Its precursor is an inactive enzyme called trypsinogen. |
| Activation | Its precursor is activated with the help of Trypsin. | Its precursor is activated with the help of enterokinase. |
| Amino Acids | It selects amino acids including tyrosine, tryptophan, and phenylalanine. | It selects amino acids including arginine and lysine. |
| Uses | It can be used for peptide mapping, peptide synthesis, analysis, and even fingerprinting. | It can be used for tissue dissociation, mitochondrial isolation, and cell harvesting. |
| Inhibitors | Its inhibitors include benzamidine, aprotinin, DFP, EDTA, Ag+, etc. | Its inhibitors include boronic acids, peptidyl aldehydes, coumarin derivatives, etc. |
What is Chymotrypsin?
Chymotrypsin is a digestive enzyme that was first discovered in the 1900s. It is part of the serine protease family and falls under the category of endopeptidases.
The enzyme has a molecular mass of 25.6 kDa. Its precursor is called chymotrypsinogen. This is an inactive enzyme that activates with the help of trypsin.
When this happens, chymotrypsin is formed and released from the pancreas as a constituent of pancreatic juice.
Every enzyme has an active site inside it that is built for particular structures and sizes to fit inside. This means the enzyme needs to select specific amino acids that can fit inside them.
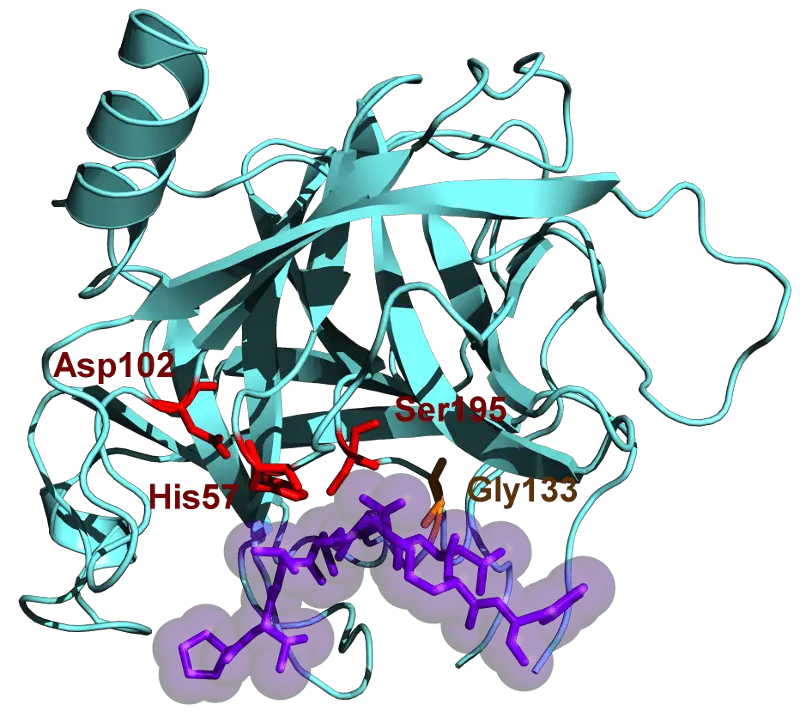
In the case of chymotrypsin, only aromatic amino acids are selected.
These include tyrosine, tryptophan, and phenylalanine. Once they enter the enzyme’s active site, their peptide bonds are broken so that they can be digested.
These enzymes serve a variety of purposes in medical and Vito studies. They are used for peptide mapping, peptide synthesis, analysis, and even fingerprinting.
There are certain inhibitors as well that bind with chymotrypsin so as to decrease its activity. These include benzamidine, aprotinin, DFP, EDTA, Ag+, etc.
They are found in supplements that are prescribed to people with dysfunctional chymotrypsin enzymes.
What is Trypsin?
Trypsin is another digestive enzyme, but it was discovered way before, in the year 1876. It also belongs to the serine proteases family but falls under the category of globular proteins.
The molecular mass of trypsin is 23.3 kDa. Its function is to break peptide bonds in amino acids.
The enzyme is released from the pancreas from its precursor called trypsinogen.
This inactive enzyme comes in contact with enterokinase for activation. Once this happens, it is carried to the small intestine, where most of its functioning takes place.
The enzyme only selects particular basic amino acids into its active site. These include arginine and lysine.
Trypsin has various uses in tissue dissociation, mitochondrial isolation, and cell harvesting. It also has several inhibitors, including boronic acids, peptidyl aldehydes, coumarin derivatives, etc.
These are found in various supplements that have numerous medical uses.
Trypsin is of two main types. These include alpha-trypsin and beta-trypsin. Each of them has a different structure and functions at a different thermal stability threshold.
However, both of their active sites contain Aspartic acid, Histidine, and Serine, which aid in the entire process of breaking amino acids. They do so by cleaving the c-terminal end, which has carbon on it.

Main Differences Between Chymotrypsin and Trypsin
- Chymotrypsin was discovered in the 1900s, whereas trypsin was discovered in 1876.
- Chymotrypsin is a digestive enzyme that breaks down aromatic amino acids, whereas trypsin is a digestive enzyme that breaks down basic amino acids.
- Chymotrypsin selects amino acids, including tyrosine, tryptophan, and phenylalanine, whereas trypsin selects amino acids, including arginine and lysine.
- The precursor of chymotrypsin is an inactive enzyme called chymotrypsinogen, whereas that of trypsin is an inactive enzyme called trypsinogen.
- The precursor of chymotrypsin is activated with the help of Trypsin, whereas that of trypsin is activated with the help of enterokinase.
- Chymotrypsin can be used for peptide mapping, peptide synthesis, analysis, and even fingerprinting, whereas trypsin can be used for tissue dissociation, mitochondrial isolation, and cell harvesting.
- Chymotrypsin inhibitors include benzamidine, aprotinin, DFP, EDTA, Ag+, etc., whereas trypsin inhibitors include boronic acids, peptidyl aldehydes, coumarin derivatives, etc.
- https://www.sciencedirect.com/science/article/pii/0022283672900289
- https://www.sciencedirect.com/science/article/pii/0014579395014845

Excellent detailed comparison of the two enzymes. Very informative and well-written.
Yes, it’s a very informative article. Well-explained differences between the two enzymes.
I learned a lot from this. The precise information makes it easy to understand.
This article provides a great insight into Chymotrypsin and Trypsin. Truly educational.
Very well researched and informative. A must-read for anyone interested in this topic.
I found this article very enlightening and clear. I had no idea there was such a difference between these two enzymes.
I agree, a very thorough comparison between Chymotrypsin and Trypsin.
Yes, the precision of the article makes it a very informative read.
The detailed comparison of these two digestive enzymes is fascinating. A very enlightening read.
The detailed breakdown of both enzymes is very clear and helpful!
Agree, the article is well structured and easy to follow.
Yes, a great source of information on this topic.
A well-researched piece of writing. Thanks for sharing!
Thank you for this comprehensive look at the differences between Chymotrypsin and Trypsin.