While studying the branch of Organic chemistry, you might have come across the terms- ‘Acetone’ and ‘Acetic Acid’. However, there is an academic question on differentiating the two.
They are both colourless liquids, having different types of smells. They are also used in different industries and have their separate ways of preparation.
Key Takeaways
- Acetone is a colorless, highly flammable, and volatile organic compound used as a solvent, while Acetic acid is a weak organic acid that gives vinegar its sour taste.
- Acetone is used as a solvent in many industries, such as nail polish removers, whereas Acetic acid is used to manufacture plastic and other chemicals.
- Acetone has a distinctive and flammable smell, whereas Acetic acid has a pungent and corrosive smell.
Acetone vs Acetic Acid
The difference between acetone and acetic acid lies in their composition and formula. While acetone is made up of a molecule of ketone, acetic acid is considered to be made up of a molecule of carboxylic acid.

Comparison Table
| Parameters of Comparison | Acetone | Acetic Acid |
|---|---|---|
| Formula | The chemical formula of Acetone is CH3COCH3 | The chemical formula of acetic acid is CH3COOH |
| Smell | Acetone has a smell that resembles that of a fruit. | Acetic acid has a smell similar to that of vinegar. |
| Molecule type | Acetone comes from a type of ketone molecule | Acetic acid comes from a type of carboxylic acid. |
| Molecular weight | The molecular weight of acetone is 58.08 g/mol. | The molecular weight of acetic acid is 60.052 g/mol |
| pH value | Acetone is basic. That is why its pH value is 7 | Acetic acid is acidic, and its pH value is 2.4. |
What is Acetone?
Acetone, or propanone, is a chemical liquid without any colour.
Acetone has a huge role to play in Organic Chemistry. It is volatile and also smells like that of a fruit. It plays the role of a solvent and can be mixed with water.
It comes from the group of ketones with the chemical formula- (CH3)2CO.
It has a wide range of uses in the industry. It aids in manufacturing plastic and can even be held responsible for various household works. You might have heard the name in nail polish removers.
Acetone is indeed the key ingredient in removing polishes from your nails.
It is also used to make cosmetics and other products in our daily household.
The human body can also produce acetone on its own by metabolism. Human urine and blood contain Acetone.
Since acetone is light and can quickly evaporate, it is used for cleaning. You can quickly dab it with a thin cloth or cotton; the solution is ready!
It has various uses in the textile as well as the automobile industry too. Wooden furniture and automobiles are finished with a coating called lacquers. The key ingredient for these lacquers is acetone itself.
It is not as toxic as some chemicals. But, we do have to maintain some safety precautions while working with it. Being inflammable, you must take care of it while working with acetone.
Also, you might cough if you accidentally smell acetone.
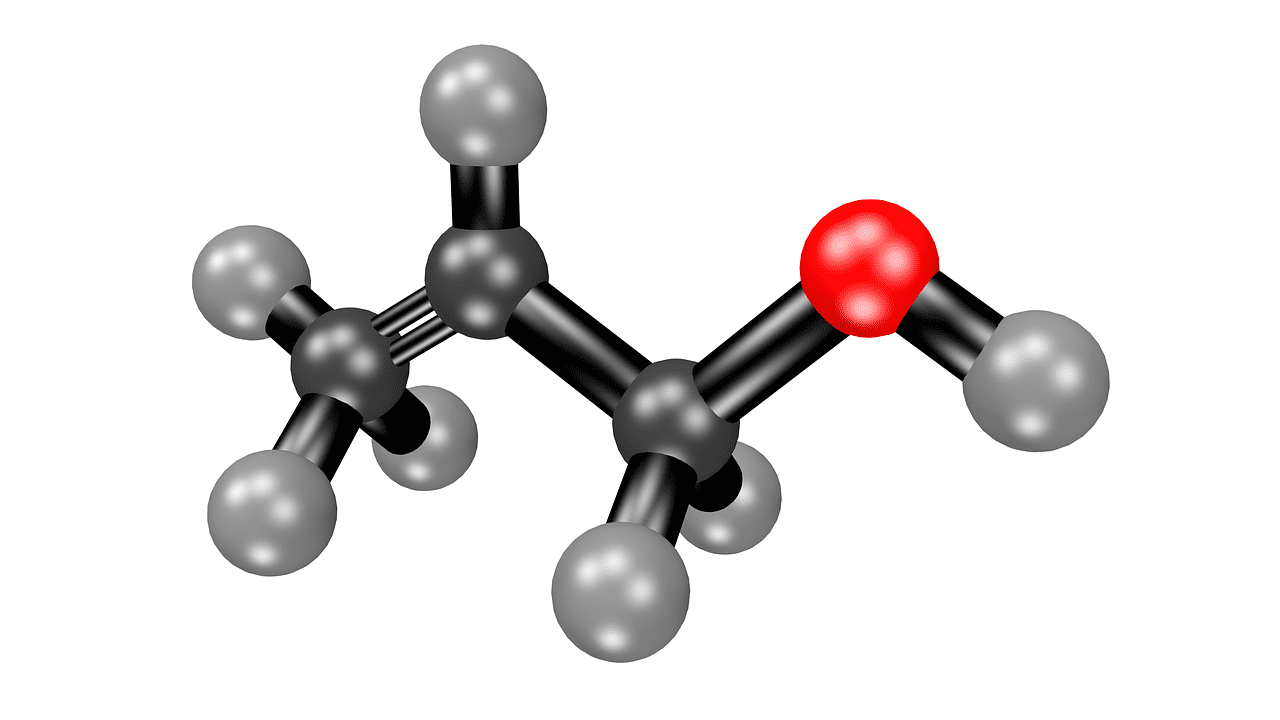
What is Acetic Acid?
Acetic acid is present throughout nature. You might be able to find acetic acid in both animals and plants as well.
Like Acetone, it is quite important in Organic Chemistry, and CH3COOH is the chemical formula of Acetic Acid. IUPAC, or the more formal name of Acetic acid, is Ethanoic Acid.
It is sour and has a smell like that of vinegar. Usually, these two factors also act as identifiers in the case of acetic acid.
It comes from a carboxylic acid and is stated as the simplest one in the family.
Industrially it is prepared from Methanol by a process called carbonylation.
Though it does not have any colour like acetone- the smell of vinegar is how we can tell it apart.
Acetic Acid has many properties for which it is useful in various fields. Initially, it can kill germs and bacteria, for which it works as an antiseptic. It is also antibacterial.
An industrial use would be the help of acetic acid in preparing fibres, especially rayon fibres. You can even use acetic acid in the treatment of cancer cells. Doctors use acetic acid to inject it into the damaged cells.
Vinegar is essentially acetic acid. For this reason, acetic acid is used in the preservation of vegetables. The key ingredient in the preparation of rubbers also happens to be acetic acid.
Several perfumes also contain acetic acid in their proportions.
Vinyl acetate monomer is produced with the help of acetic acid.
It can be mixed in the water pretty quickly. It also reacts with both polar as well as non-polar substances. Being used in various industries- Acetic Acid is a very important chemical in everyday life.

Main Differences Between Acetone and Acetic Acid
- Acetone is a chemical substance that originates from a ketone, and CH3COCH3 is the chemical formula of this substance. Acetic acid also happens to be a chemical substance, but it originated from a carboxylic acid. CH3COOH is the chemical formula of this substance.
- Both of these chemicals can be found in nature. They are either produced in the human body or by nature by certain bacteria. Acetone can be created in the human body via metabolism. When fats are broken down, acetic acid is produced. On the other hand, acetic acid can be produced by a bacteria called Acetobacter via fermentation.
- Both substances can also be produced in a lab. There are artificial means to produce these two. Making acetic acid in an artificial method is known as cumene hydroperoxide. On the other hand, acetic acid can be produced by the fusion of carbon dioxide and methanol.
- Acetone removes paint from nails, while acetic acid can make plastics.
- Acetone can also be used in the stripping of paints industrially. On the other hand, acetic acid is used to preserve vegetables by pickling.

I wonder if we should be more careful handling these chemicals. It seems they can be risky as well.
It’s true! It’s always important to practice safety measures when handling these chemicals.
I think the risks should not be overlooked.
The article provides a great comparison of acetone and acetic acid, very well detailed.
Yes, details are very well explained here.
Completely agree, the details are very informative.
This article provides an informative comparison between acetone and acetic acid. It’s beneficial to understand their differences.
I couldn’t agree more.
Absolutely, It’s always good to have a clear understanding of these compounds.
I think it’s funny how the human body produces acetone and the relevance of it to our household products.
It’s indeed quite amusing!
Acetone & acetic acid are essential chemicals in many industries. It’s amazing to see how versatile they are!
They really are, there’s a lot more to these chemicals than one would think.
Absolutely! The versatility of these chemicals is fascinating.
The informative content helps to understand the uses and potential hazards of acetone and acetic acid.
It’s essential to be aware of the hazards of these chemicals to be cautious.
Absolutely, the importance of proper use of these chemicals cannot be overstated.
This post does a good job of detailing the different qualities and uses of acetone and acetic acid.
Quite an informative post on the subject.
Yes, a detailed comparison of these chemicals.
The article is a good source for understanding the properties and uses of acetone and acetic acid.
Indeed, a great read for those interested in chemistry.
Very informative, indeed.
Quite an interesting article on these organic compounds, a good read.
An interesting and informative article for sure.
Indeed, a good read.
It’s interesting to see how these two organic compounds are used differently despite their similarities. They seem rather harmless given their various uses.
Yes, these compounds are really interesting to study!
Totally agreed!