Chemistry is a wide concept with various compounds and elements. Acetone and Xylene are organic compounds with specific characteristics and structures. Acetone is represented with the chemical formula C3H6O, while C8H10 represents xylene.
Both of these chemical compounds are colorless, flammable, and toxic substances that manufacturers use in producing various industrial products.
Key Takeaways
- Acetone is a colorless, volatile solvent with a distinct odor, while xylene is an aromatic hydrocarbon with a sweet scent.
- Acetone evaporates quickly and is less toxic, while xylene evaporates slower and poses greater health risks.
- Acetone works well as a general-purpose solvent, while xylene dissolves and cleans oil-based materials.
Acetone vs Xylene
The difference between acetone and xylene is that acetone is an organic compound presented with the chemical formula C3H6O. It is a colorless flammable liquid substance with a fruity smell. It is also called propanone and is used in various industrial products. On the contrary, xylene is also an organic compound presented with the chemical formula C8H10. In the structure, this compound has a benzene ring. It is a flammable substance and insoluble in nature.

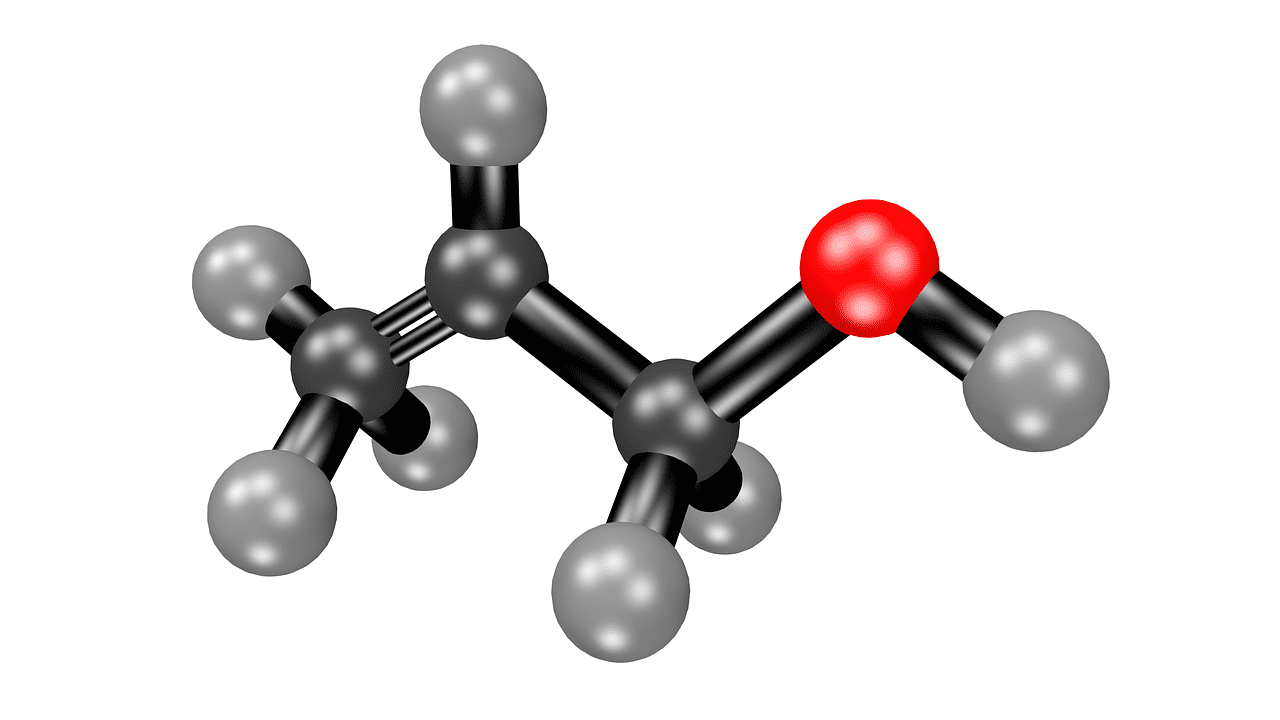
Acetone is a hydrocarbon organic compound with the chemical formula C3H6O. This compound is primarily used in cosmetics, including nail polish removers. It is a flammable substance that evaporates easily and quickly.
This compound is colorless with a fruity smell.
Xylene is also an organic compound with the chemical formula C8H10. It is colorless, toxic, and flammable in nature. This organic compound is effectively used in industries and used as a solvent.
Xylene is prepared in a chemical reaction that includes benzene and toluene.
Comparison Table
| Parameters of Comparison | Acetone | Xylene |
|---|---|---|
| Definition | Acetone is a colorless organic compound represented with the chemical formula C3H6O | Xylene is an organic compound represented with the chemical formula C8H10 |
| Molecular Weight | Molecular weight of acetone is 58.08 g/mol | Molecular weight of xylene is 106.16 g/mol |
| Oxygen atom | This organic compound has one oxygen atom | This organic compound does not have any oxygen atom |
| Odor | It has a fruity smell | It has a sweet smell |
| Molecule Type | Acetone has ketone structure | Xylene has hydrocarbon structure |
| Formation | It is manufactured by using cumene hydroperoxide procedure | It is manufactured by using petroleum |
| Uses | It is used in the nail polish remover as a solvent and also used to remove lacquers | It is used as a cleaning agent and in the leather and paints industry as a solvent |
What is Acetone?
Acetone is a chemical compound that is also called dimethyl ketone and propanone, presented with the formula (CH3)2C6H4. This compound is colorless and flammable with a fruity smell.
It is used in various industrial products, including cosmetics, nail polish removers, artificial fibre manufacturing, etc. Acetone is commercially produced by using the cumene hydroperoxide process and dehydrogenation.
Acetone is a useful compound used at an appropriate level or amount to manufacture products that dissolve other products.
For industry purposes, this compound is used effectively to produce plastics and lacquers and remove grease from various textiles. In the human body, fat breakdown into acetone, and hence it produces as a byproduct naturally.
What is Xylene?
Xylene is a colorless organic compound presented with (CH3)2CO, also known as xylol, with a molecular weight of 106.16 g/mol. It is an insoluble and toxic liquid that is flammable in nature.
This compound is formed naturally and by industrial preparation. Naturally, it is produced in crude oil, including aircraft fuels and gasoline. It can be used as a solvent and have a sweet smell.
For industrial purposes, xylene is used by manufacturers as a raw material for making dyes and fibres. It is used as a clearing agent and in laboratories as well to cool the vessels in which reactions are incurred.
Xylene is the result of an alkylation reaction that includes benzene and toluene. It is a toxic and dangerous chemical compound that can affect even the nervous system and organs like the kidneys, liver, and lungs.
Main Differences Between Acetone and Xylene
- Acetone and xylene are organic compounds that are presented with different chemical formulas. Acetone is a chemical compound that is also called dimethyl ketone and propanone, presented with the chemical formula C3H6O while xylene, on the other side, is presented with the chemical formula C8H10.
- Every chemical compound has a different structure and molecular arrangement. The molecular weight of acetone is 58.08 g/mol. On the contrary, the xylene molecule is comparatively bigger than acetone, and hence it has a relatively higher molecular weight, 106.16 g/mol.
- Acetone and xylene are colorless flammable organic compounds with a specific odour. The smell of acetone compound is similar to that of a fruit or in other words, it has a fruity smell, but xylene, on the other side, has a sweet smell.
- The molecule in the acetone formed a ketone structure that contains one oxygen atom. This compound is manufactured by using the cumene hydroperoxide procedure. While xylene, on the other side, has a hydrocarbon structure, it does not contain any oxygen atoms and is manufactured by using an alkylation reaction that includes benzene and toluene.
- Acetone and xylene have various uses. Acetone is used effectively to produce plastics, lacquers and remove grease from various textiles. On the contrary, Xylene is used as a cleaning agent and in the leather and paints industry as a solvent.





