A chemical element comprises several units with independent properties, functions, and chemistry with different units of their types or another.
Because of these small segments, an element has unique roles, aspects, and usages. Two of them are- 1. Atom 2. Ion.
Key Takeaways
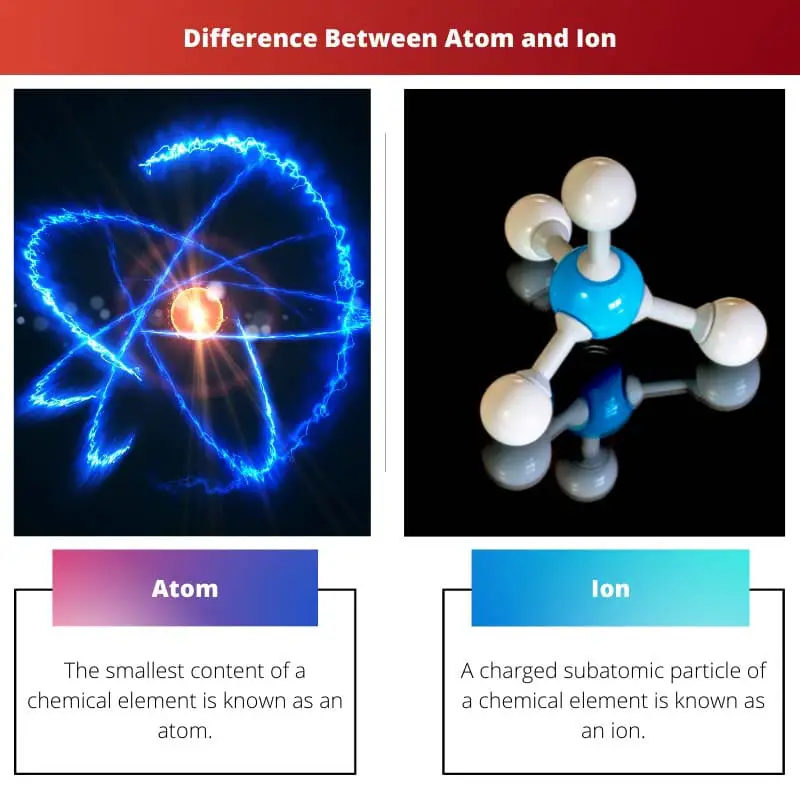
- An atom is the smallest constituent unit of matter that retains the chemical properties of an element. In contrast, an ion is a charged particle that forms when an atom loses or gains electrons.
- Atoms have a neutral charge, and the number of protons in the nucleus determines their atomic number and chemical properties. In contrast, ions have a positive or negative charge and have different chemical properties than their neutral atoms.
- Atoms can combine with other atoms to form molecules. In contrast, ions can combine with other ions or atoms to form ionic compounds, and their properties are determined by their charge and size.
Atom vs Ion
An atom is a basic unit of matter that consists of a nucleus (made up of protons and neutrons) and electrons that orbit the nucleus. An ion is an atom or molecule with unequal protons and electrons, giving it a net electrical charge. Atoms can become ions through ionization, where they gain or lose electrons.

The most negligible content of a chemical element is called an atom which constitutes protons, electrons, and neutrons. Almost all the mass of an atom is responsible because of the nucleus.
Particles with a positive charge in an atom are called protons, particles with a negative charge are called electrons, and those with no charge are called neutrons.
An ion is part of a chemical element that has a net electric charge. An ion doesn’t have an equal number of electrons and protons, so it possesses a net electric charge.
An ion consists of (1) cation and (2) anion. An ion with a positive charge which contains limited electrons is called a cation.
An ion with a negative charge which contains surplus electrons is called an anion. Cations and anions are oppositely charged; hence, they attract each other, and their resultant product is an ionic compound.
Comparison Table
| Parameters Of Comparison | Atom | Ion |
|---|---|---|
| Definition | The most negligible content of a chemical element is known as an atom. | A charged subatomic particle of a chemical element is known as an ion. |
| First discovered in | 450 B.C. | 1834 |
| First discovered by | Democritus | Michael Faraday |
| Terminology | Derived from an ancient Greek word “ἰόν.” | Derived from an ancient Greek word “ἰόν” |
| Examples | Neon, Hydrogen, Oxygen, Argon, Iron, Calcium, Fluorine, Chlorine, Sodium, Plutonium, Deuterium, Carbon, Sulphur, Bromine, Iodine, Potassium, Copper, Boron, Lithium, Cobalt, Nickel | monoatomic ions- F−, Cl−, Br−, I−, Li+ Polyatomic ions- SO42–, CO32− Ionic compounds- sodium chloride, potassium chloride |
| Bibliographical references | “From atomos to atom: the history of the concept of the atom” by Andrew G. van Melsen, “Ernest Rutherford and the explosion of atoms” by John L. Heilbron, “A history of the electron” by Jaume Navarro | “Earth” 14th edition by Frank Press and Raymond Siever, “Radiation Detection and Measurement” by Glenn Knoll |
What is Atom?
The most negligible content of a chemical element or the smallest particle of matter is called an atom. The main contents of an atom are protons, neutrons, and electrons.
Protons have a positive charge. Electrons have a negative charge. Neutrons have no charge. Examples of an atom are Hydrogen, Oxygen, Scandium, Lead, Copper, Mercury, Sodium, Uranium, Krypton, Xenon, Barium, and Sulphur.
Several scientists state theories and postulates regarding an atom’s physical and chemical properties, nature, behaviour, and other parameters.
John Dalton, an English chemist, discovered and stated the currently known “law of multiple proportions” theory wherein he inferred that several chemical elements consist of different ratios of mass, due to which their quantity in a chemical compound is different.
It was because of Sir J.J. Thomson that the world got familiar with the fact that an atom also has other contents, namely, the nucleus.
His discovery of the nucleus was based on a “plum pudding model,” which made it inevitable that an atom consists of a nucleus along with electrons, protons, and neurons.
Although, Ernest Rutherford, with his epiphanies regarding an atom, overcame the difficulties found in Thomson’s atomic model.
The history of the atom and various discoveries regarding it were initiated long back in Greek and Indian ancient cultures. After that, various discoveries about it, such as the law of multiple proportions, the Kinetic theory of gases, Brownian motion, and the Discovery of the nucleus, neutron, isotopes, and electron, were possible.
Because of these remarkable and improved theories, the world is familiar with all sorts of information regarding the atom.
An atom has several properties with parameters such as nuclear properties, mass, shape, size, magnetic moment, energy levels, valence, and bonding behaviours with other atoms, states, etc.
According to the Modern Periodic Table, Hydrogen is an element with the least number of atoms.
What is Ion?
A charged subatomic particle of a chemical element is known as an ion. There are several subcategories of an ion. Based on their charge, the two main types of ions are- cations and anions.
Cations have a positive charge. On the other hand, anions have a negative charge.
Furthermore, it is categorized based on the number of atoms present. Ions with a single atom are called monoatomic ions.
On the contrary, polyatomic ions have two or more atoms. Both polyatomic and monoatomic ions can either be cations or anions.
Since they are oppositely charged, they attract each other and form an ionic bond, and the resultant product is an ionic compound.
Examples of monoatomic ions are F−, Cl−, Br−, I−, Li+, Na+, and Rb+. Examples of polyatomic ions are SO42–, CO32−, CrO42-, PO43-, BO33-.
Examples of ionic compounds are Pottasium chloride, Sodium chloride, Calcium oxide, Magnesium sulfide, Sodium phosphide, Lithium acetate, Silver bromide, and Silver nitrate.
Michael Faraday and his correspondence William Whewell made the first discovery regarding an ion in 1834.
Faraday did not know the nature of an ion back then, but he did believe that an ion requires an aqueous medium to travel from one electrode to the other.
Whewell was the one to coin the terms cathode, anode, cations, and anions.
Another key person in the history of ion is Svante Arrhenius. In his hypothesis, in 1884, Arrhenius stated the justification for dissociating solid crystalline salts into paired charged particles.
He also believed that ions are formed despite the absence of an electric current.
There are several properties of ions, such as common ion effect, degree of ionization, ionization, ionization potential, ionic bond, inorganic ions, ionic transfer, electrode ionization, quinonoid zwitterion, tunnel ionization, etc.
Ions have several dailies and industrial applications, such as an indication of water quality and air purification; they are used in smoke detectors, etc.
Main Differences Between Atom and Ion
- Atom is electrically neutral. On the other hand, an ion has either a positive or a negative charge.
- Ions have more applications, whereas atoms have lesser applications.
- Ions have more properties than atoms.
- The mass of an atom can be calculated. On the other hand mass of an ion cannot be calculated, but a mass of an ionic compound can be calculated.
- The formula used for calculating an ionic compound’s mass is the sum of the atomic masses of the ions present in the formula, whereas the formula used to calculate atomic mass is the sum of atoms in the molecule.
- https://books.google.com/books?hl=en&lr=&id=Yy0LAAAAIAAJ&oi=fnd&pg=PR5&dq=history+of+atoms&ots=0mattvVEVk&sig=xYchnIcl8MoSkKQ26xFgZKXgpAo
- https://books.google.com/books?hl=en&lr=&id=5JnzCAAAQBAJ&oi=fnd&pg=PA1&dq=atoms+ions&ots=WypZZNdo2D&sig=N2fZHMxHYgpcQtNsROVFZ80AG_s