Ionic compounds are formed through the transfer of electrons between atoms, resulting in charged ions held together by electrostatic forces. Molecular compounds, on the other hand, are composed of covalently bonded atoms, sharing electrons to form discrete molecules.
Key Takeaways
- Ionic compounds are composed of ions that are held together by electrostatic forces.
- Molecular compounds are composed of molecules that are held together by covalent bonds.
- Ionic compounds have higher melting and boiling points than molecular compounds and are soluble in water.
Ionic Compounds vs Molecular Compounds
Ionic compounds are formed by the ionic bonds in which the atoms are electrostatically attracted to one another. They have interaction of cations and anions in them. While molecular compounds are formed by covalent bonds, in which the electrons are shared by the atoms forming the bond.
To understand the difference better, you need to understand the basic terminology well. Two or more two atoms of different elements combine to form a molecule, which is the basic unit of a compound.
Every compound is different in terms of properties. This is due to the fact that every element that a compound consists of possesses different properties. Electronegativity is also one of the most important terms to know.
Electronegativity is the tendency of an atom of an element to attract the electrons of other elements towards its core. A compound may be polar or non-polar, and this entirely depends on the electronegativity of the elements.
Comparison Table
| Feature | Ionic Compounds | Molecular Compounds |
|---|---|---|
| Formation | Formed by the transfer of electrons between a metal and a nonmetal, resulting in oppositely charged ions (cations and anions) attracting each other. | Formed by the sharing of electrons between two or more nonmetals, forming covalent bonds to hold the atoms together. |
| Bonding Type | Ionic bonding (electrostatic attraction between oppositely charged ions) | Covalent bonding (sharing of electrons between atoms) |
| Structure | Crystalline lattice structure, with a regular arrangement of cations and anions. | Discrete molecules, with specific shapes and arrangements of atoms. |
| State at Room Temperature | Typically solids | Can be solids, liquids, or gases depending on the compound. |
| Electrical Conductivity | Good conductors in molten or aqueous state, as ions can move freely. | Poor conductors in all states, as the electrons are tightly bound within molecules. |
| Solubility in Water | Generally soluble in water due to the attraction of ions to water molecules. | Varying solubility in water, depending on the polarity and size of the molecule. |
| Examples | Sodium chloride (NaCl), Calcium oxide (CaO), Potassium sulfate (K₂SO₄) | Water (H₂O), Carbon dioxide (CO₂), Methane (CH₄) |
What are Ionic Compounds?
Ionic compounds are a type of chemical compound characterized by the presence of ions, which are atoms or groups of atoms that have gained or lost electrons, resulting in a net electrical charge. These compounds typically form when atoms of metals react with atoms of nonmetals, leading to the transfer of electrons from the metal to the nonmetal.
Formation of Ionic Compounds
The formation of ionic compounds involves the process of ionization, where atoms either gain or lose electrons to achieve a stable electronic configuration. Typically, metals tend to lose electrons to form positively charged ions known as cations, while nonmetals tend to gain electrons to form negatively charged ions called anions.
For example, in the formation of sodium chloride (NaCl), sodium (Na) atoms, with one electron in their outermost shell, lose this electron to achieve a stable electron configuration of neon, forming Na⁺ ions. Conversely, chlorine (Cl) atoms, requiring one electron to complete their outermost shell, gain this electron to form Cl⁻ ions. The resulting attraction between the oppositely charged ions leads to the formation of an ionic bond.
Characteristics of Ionic Compounds
- Crystal Lattice Structure: Ionic compounds typically form a three-dimensional lattice structure, where each cation is surrounded by anions and vice versa. This arrangement maximizes the attraction between oppositely charged ions, resulting in strong electrostatic forces holding the lattice together.
- High Melting and Boiling Points: Due to the strong electrostatic forces between ions, ionic compounds generally have high melting and boiling points. This is because a considerable amount of energy is required to overcome these forces and break the bonds holding the lattice together.
- Solubility in Water: Many ionic compounds are soluble in water due to the polar nature of water molecules. When an ionic compound dissolves in water, the water molecules surround individual ions, effectively separating them from the crystal lattice and allowing them to disperse throughout the solution.
- Conductivity: In the solid state, ionic compounds do not conduct electricity because the ions are held in fixed positions within the lattice structure. However, when dissolved in water or melted, the ions become free to move and can conduct electricity, making molten ionic compounds and their aqueous solutions good conductors of electricity.
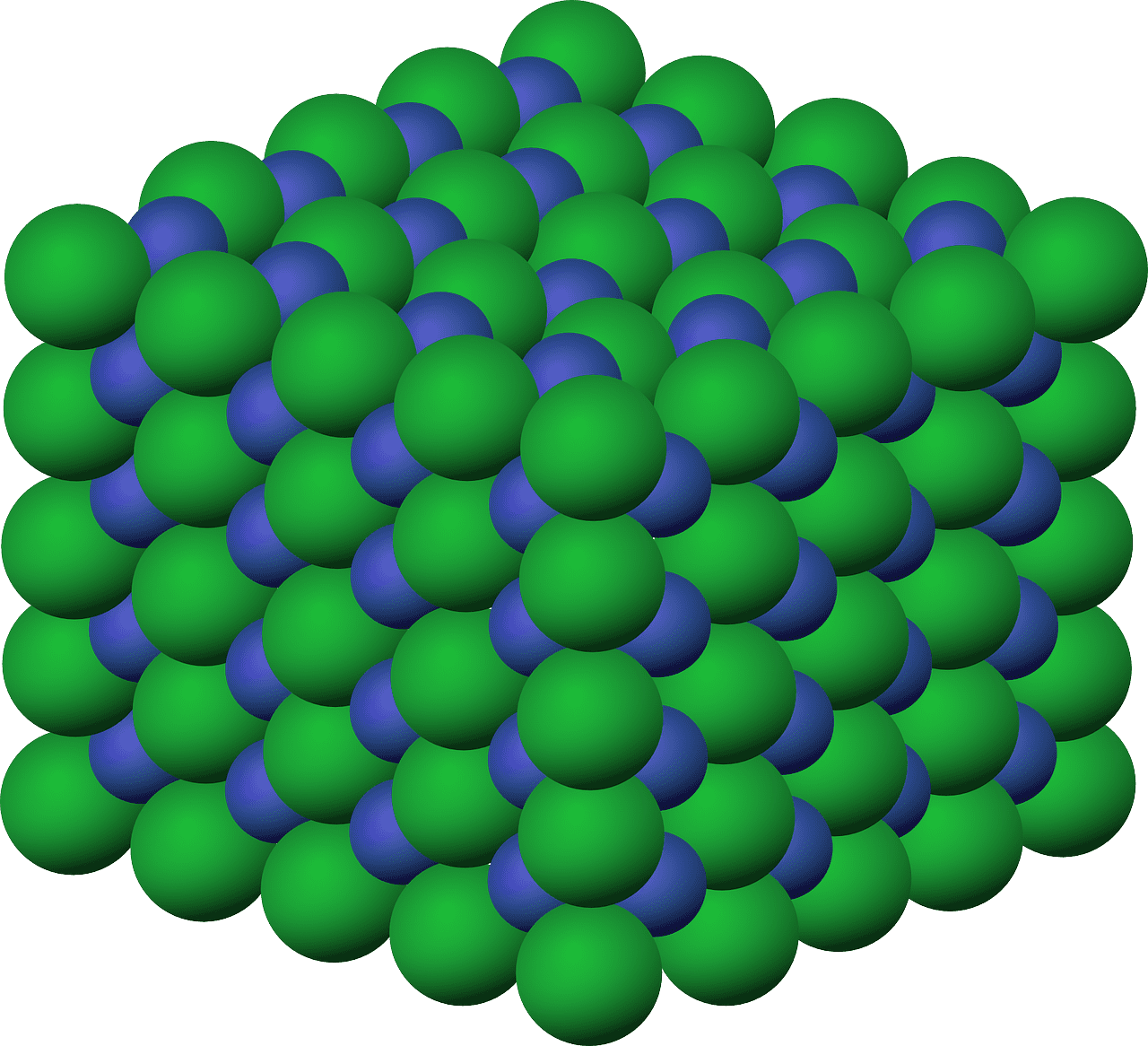
What are Molecular Compounds?
Molecular compounds are chemical compounds composed of molecules formed through the sharing of electrons between atoms, primarily through covalent bonds. Unlike ionic compounds, which involve the transfer of electrons leading to the formation of ions, molecular compounds consist of discrete units called molecules, where atoms are held together by shared pairs of electrons.
Formation of Molecular Compounds
Molecular compounds form when atoms of nonmetals bond together by sharing electrons to achieve a stable electron configuration. In a covalent bond, atoms share one or more pairs of electrons, resulting in the formation of a molecule. The sharing of electrons allows each atom to attain a full outer shell, typically consisting of eight electrons (octet rule), or two electrons for hydrogen.
For example, in the formation of water (H₂O), two hydrogen (H) atoms each share a pair of electrons with one oxygen (O) atom. This sharing of electrons creates covalent bonds between the hydrogen and oxygen atoms, resulting in the formation of a water molecule.
Characteristics of Molecular Compounds
- Low Melting and Boiling Points: Molecular compounds generally have lower melting and boiling points compared to ionic compounds. This is because the intermolecular forces between molecules (such as van der Waals forces or hydrogen bonds) are weaker than the ionic bonds present in ionic compounds.
- Varied Solubility: The solubility of molecular compounds in water varies depending on the polarity of the molecules. Polar molecules tend to dissolve in polar solvents like water, while nonpolar molecules dissolve better in nonpolar solvents. This solubility behavior is due to the interactions between the polar or nonpolar regions of molecules and the solvent molecules.
- Existence in Multiple Phases: Molecular compounds can exist in different phases (solid, liquid, or gas) under standard conditions, depending on factors such as molecular size, shape, and intermolecular forces. For instance, some molecular compounds, like water, can exist in all three phases depending on temperature and pressure.
- Non-Conductivity: Molecular compounds generally do not conduct electricity in any state (solid, liquid, or gas) because they do not contain free ions or mobile charged particles. Electric current requires the presence of charged particles, which are absent in molecular compounds where electrons are shared rather than transferred.
Main Differences Between Ionic Compounds and Molecular Compounds
- Bonding Mechanism:
- Ionic compounds form through the transfer of electrons, resulting in the formation of ions and electrostatic attraction between oppositely charged ions.
- Molecular compounds form through the sharing of electrons between atoms, resulting in the formation of discrete molecules held together by covalent bonds.
- Composition:
- Ionic compounds are composed of ions, which are atoms or groups of atoms with a net electrical charge.
- Molecular compounds are composed of molecules, which are groups of atoms held together by covalent bonds.
- Physical Properties:
- Ionic compounds often have high melting and boiling points due to strong electrostatic forces between ions.
- Molecular compounds typically have lower melting and boiling points compared to ionic compounds due to weaker intermolecular forces between molecules.
- Conductivity:
- Ionic compounds conduct electricity when dissolved in water or melted due to the presence of free ions capable of carrying electrical charge.
- Molecular compounds generally do not conduct electricity in any state (solid, liquid, or gas) because they do not contain free ions or mobile charged particles.
- Solubility:
- Many ionic compounds are soluble in water due to the polar nature of water molecules, which can surround and dissociate ions from the crystal lattice.
- The solubility of molecular compounds varies depending on the polarity of the molecules, with polar molecules dissolving in polar solvents and nonpolar molecules dissolving in nonpolar solvents.

Last Updated : 06 March, 2024

Piyush Yadav has spent the past 25 years working as a physicist in the local community. He is a physicist passionate about making science more accessible to our readers. He holds a BSc in Natural Sciences and Post Graduate Diploma in Environmental Science. You can read more about him on his bio page.

I wish chemistry was taught like this in school, it would make the subject much easier to understand.
Absolutely, this was a great read.
I agree, the comparisons and explanations were really helpful.
The article covers the topic thoroughly, providing a comprehensive overview of ionic and molecular compounds.
Well-articulated and research-based, an excellent piece of work.
The comparisons between ionic and molecular compounds are very enlightening.
The article provides a balanced and well-elaborated account of ionic and molecular compounds.
The content is thoughtfully presented and thoroughly researched, great job!
The detailed comparison of the properties and characteristics of ionic and molecular compounds is both enlightening and engaging.
The article provides a clear and systematic analysis of ionic and molecular compounds.
Yes, the comparison table is very helpful in highlighting the distinctions between the two compound types.
I find the information presented here quite helpful in understanding the fundamental differences between ionic and molecular compounds.
The content is well-explained, making it accessible to a wide range of readers.
Absolutely, the article is a great resource for students studying chemistry.
A comprehensive comparison. Very well-structured and informative.
I couldn’t have explained it better myself, great job!
The explanations are clear and concise, making it easy to grasp the concepts presented.
The description of the properties and differences between ionic and molecular compounds is excellent.
A very informative piece, well done!
This was a very educational explanation, thank you!
I agree, the examples of ionic and molecular compounds help solidify the concepts.
Great content, well-summarized!
The explanation of electronegativity in relation to ionic and molecular compounds is particularly insightful.
Absolutely, understanding electronegativity is fundamental to comprehending chemical bonding.
I strongly disagree with some of the points made in this article, particularly the discussion around boiling and melting points.