Insulators are used daily by all of us, from panhandles to underground pipe coating. On the other hand, semiconductor materials are mainly used in electronic devices and have great use in our electronics industries.
Key Takeaways
- Insulators are materials that do not conduct electricity well and have high resistivity, while semiconductors have moderate resistivity and conductivity.
- Semiconductors can conduct electricity under certain conditions and are used in electronic devices, while insulators are used to prevent electricity from flowing.
- The conductivity of semiconductors can be increased by adding impurities through doping, while insulators cannot be doped to increase their conductivity.
Insulator vs Semiconductor
A massive gap between the valance band and conduction band in the insulator prevents free electrons from conducting electricity. On the other side, semiconductors have less bandgap than insulators, which high energetic electrons can overcome.

Insulators are poor conductors of heat and electricity. Their resistance is very high, so electricity can’t pass through them.
They are mainly used in insulating conduction wires. They form a barrier between two conducting bodies to prevent short-circuiting and accidents.
Some common insulating materials are paper, wood, rubber plastics, etc.
Semiconductors have a moderate conductivity level. Their resistance to electricity can be varied by adding impurities in it.
This process is called doping. A small amount of added impurity can lead to a huge difference in conduction.
The semiconductors can be pure such as germanium and silicon, or compounds, such as gallium arsenide or cadmium selenide.
Comparison Table
| Parameters of comparison | Insulator | Semiconductor |
|---|---|---|
| Conductivity | < 10 -13 mho/m | Between 10 -7 to 10 -13 mho/m |
| Majority charge carriers | No conduction due to the absence of carriers | Movement of electrons and holes |
| No. of valence electrons | Their valence shell is complete, i.e. 8 electrons | They have four valence electrons in the outermost shell |
| Bandgap | There is a huge bandgap of 6eV -10eV | There is a bandgap of 1.1eV |
| Valence band | Filled | Partially empty |
| Conduction band | Empty | Partially filled |
| Absolute zero | Resistance increases | Turn into an insulator |
| Resistivity | High | Moderate |
| Example | Rubber, plastic, paper, etc | Silicon, germanium, gallium arsenide |
| Applications | Home appliances, coating of cable wires, etc | Integrated circuits, diodes, resistors, etc |
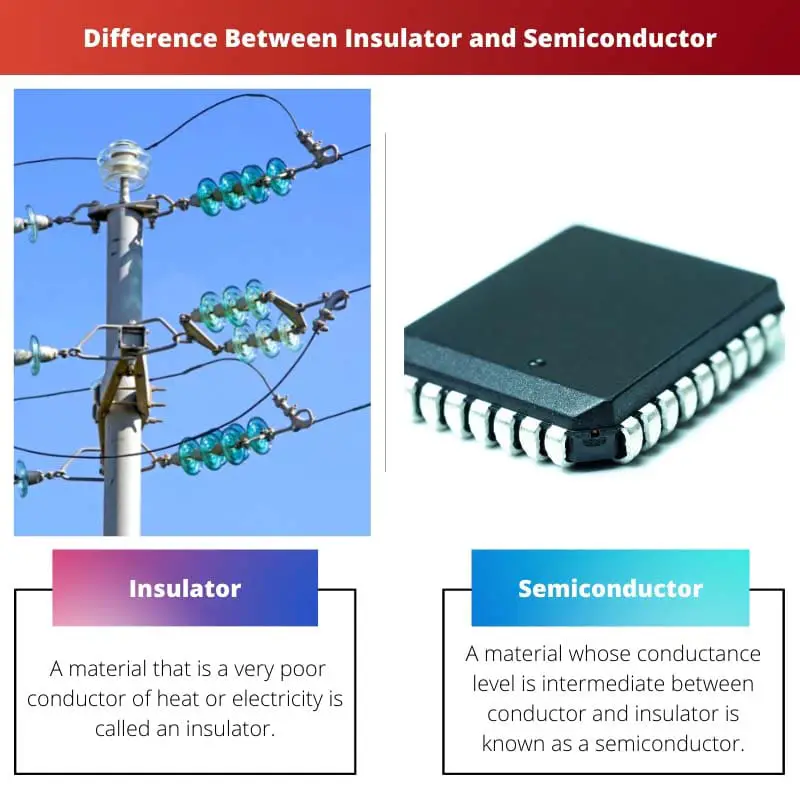
What is Insulator?
A material that is a very poor conductor of heat or electricity is called an insulator. Its conductance level is very low.
Conduction is the property of easy flow of current carries through them. Insulators have a complete valence band of 8 electrons in them.
As a result, there is an absence of free carriers to conduct electricity.
According to band theory, a huge bandgap of 6eV to 10eV does not allow electrons to jump from the valence band to the conduction band. They have a filled valence band and an empty conduction band.
They have very high resistance because of which no current can pass through them. On increasing temperature, the resistivity of an insulator decreases.
Temperature leads to loosing of covalent bonds present in them and increases the number of carriers in them.
At absolute zero temperature, the resistance of the insulator increases. There are many types of insulators, such as sound insulators, thermal insulators, and electrical insulators, depending upon the field of the use of the material.
Pin insulators are the first insulators used. A vacuum is also an insulator.
This is due to the fact of absence of carriers there. Some examples of insulators are rubber, plastic, etc.

What is Semiconductor?
A material whose conductance level is intermediate between conductor and insulator is known as a semiconductor. The conductance level can be altered by adding several impurities to the semiconductor crystal.
There are pure semiconductor crystals like silicon or germanium and compound semiconductors like gallium arsenide or cadmium selenide.
There are mainly two types of a semiconductor having huge applications in modern electronics industries. They are intrinsic semiconductors (Si and Ge) and extrinsic semiconductors (n-type and p-type).
The n-type extrinsic semiconductor is formed by adding group III elements in pure Si or Ge. These impurities are called donors.
The p-type extrinsic semiconductor is formed by adding group V elements in pure Si or Ge. These impurities are known as acceptors.
They have both types of carriers, holes and electrons, that conduct electricity. Their conductance is between 10-7 to 10-13 mho/m.
They have a moderate energy band gap covered by electrons to move to the conduction band. Their valence band is partially filled with 4 electrons. They have a covalent type of bonding.
They lose their conductance property at zero temperature and turn into insulators. They are very compact, have a long life application, and have low cost, which makes them very in demand in modern technologies.
Semiconductors have a huge application in making diodes and transistors, MOSFET, etc.
Main Differences Between Insulators and Semiconductors
- The key difference between insulators and semiconductors is their range of conductivity. The conductance of the insulator is 10-13 mho/m, whereas semiconductors have conductance between 10-7 to 10-13 mho/m.
- They have a different energy bandgap; that is, for semiconductors, it is 1.2eV, and for insulators, it is 10eV.
- Insulators do not have any carriers in them, so there is no conductivity in them, and on the other hand, semiconductors have electrons and holes to conduct.
- At absolute zero temperature, the resistance to the insulator increases, whereas the semiconductor completely loses its conductance and behaves as an insulator
- The insulators have only covalent bonding, whereas the semiconductors have both ionic and covalent bonding.
- The insulators have a complete valence shell, and semiconductors have a partially filled valence shell with 4 electrons.
- Insulators have a very high resistance which does not allow electricity or heat to flow through them. Still, semiconductors have a moderate resistance, allowing the flow to the current but sometimes blocking it.
Reference
- https://pubs.acs.org/doi/abs/10.1021/acs.nanolett.9b04187?casa_token=Udhvcpd5v4QAAAAA:JLS2H_D2xAnvWgO3b373dzQ-8TOgwXYYyKu5bszsg0-5cJpD0ZAw4JzzkdJFcCTr8JNYJym4qmUROCFQ
- https://journals.aps.org/prb/abstract/10.1103/PhysRevB.27.7509

Wonderful article. The explanation of the difference between insulators and semiconductors was very detailed and informative. I thoroughly enjoyed reading this post.
I agree completely with your assessment Zmatthews, the explanation was clear and concise. Great contribution to the topic.
I found this article to be an invaluable resource for understanding semiconductors and insulators. The descriptive explanations were extremely helpful.
I definitely agree with you, Adam Scott. The descriptive nature of the explanations made this a very enriching read.
Couldn’t have said it better, Adam Scott. The descriptive depth was truly commendable.
The detailed analysis of insulators and semiconductors was impressive. It has certainly broadened my understanding of these materials and their properties.
The applications section was particularly insightful. It’s crucial to understand the practical uses of materials like insulators and semiconductors in various industries.
Absolutely agree, Katie Green. The article provided a comprehensive and valuable overview of the real-world applications of these materials.
I second your thoughts, Katie Green and Bmarshall. The practical insights into the uses of insulators and semiconductors were a standout feature.
I’m not convinced by the comparisons made in this article. It seems that the complexities of semiconductor materials were not fully captured in this discussion.
I have to disagree with you, Ggraham. The article did an admirable job of laying out the differences between these two material types. The comparisons were very well articulated.
This post provided an insightful comparison between insulators and semiconductors. As a student of materials science, I found this to be an enriching read.
The detailed breakdown of the conductivities, bandgaps, and valence bands of insulators versus semiconductors was truly fascinating. This article has expanded my knowledge on the subject significantly.
Yes, I concur with both of you. This article has definitely raised the level of discussion on semiconductors and insulators.
I’m glad I’m not the only one who found this interesting! Sebastian Ross, your insights are quite astute. The depth of information provided was impressive.
This article was an enlightening read. The comparison table neatly summarizes the key differences between insulator and semiconductor materials.
I couldn’t agree more with you, Kieran13. The comparison table adds significant value to the post.
This article was written with utmost clarity. The definitions of insulators and semiconductors were very well-articulated, making it highly accessible to readers.
Absolutely, Caroline Hunter. The clarity of information in this post was commendable. It’s a well-crafted piece.
The content is far too simplistic. A more in-depth analysis is needed to truly appreciate the nuances between insulators and semiconductors.
I beg to differ, Butler Nick. The detailed comparison provided a nuanced understanding of these materials. The depth was certainly there.