There are different types of elements in our surroundings. These elements are organised into different categories based on their physical features like shape, size, colour, texture, polarity, malleability, solubility, etc.
One such important category based on which elements are classified is conductivity. That is the ability of a component to allow ions or electrons to move freely. Based on their conductivity, elements are categorised into conductors and insulators.
Key Takeaways
- Conductors are materials that allow the flow of electric charge, making them essential components in electrical circuits and power transmission.
- Insulators are materials that resist the flow of electric charge, providing protection from electrical currents and helping to prevent short circuits and electrical hazards.
- The choice between conductors and insulators depends on the specific application, with conductors facilitating the flow of electricity and insulators preventing it.
Conductor vs Insulator
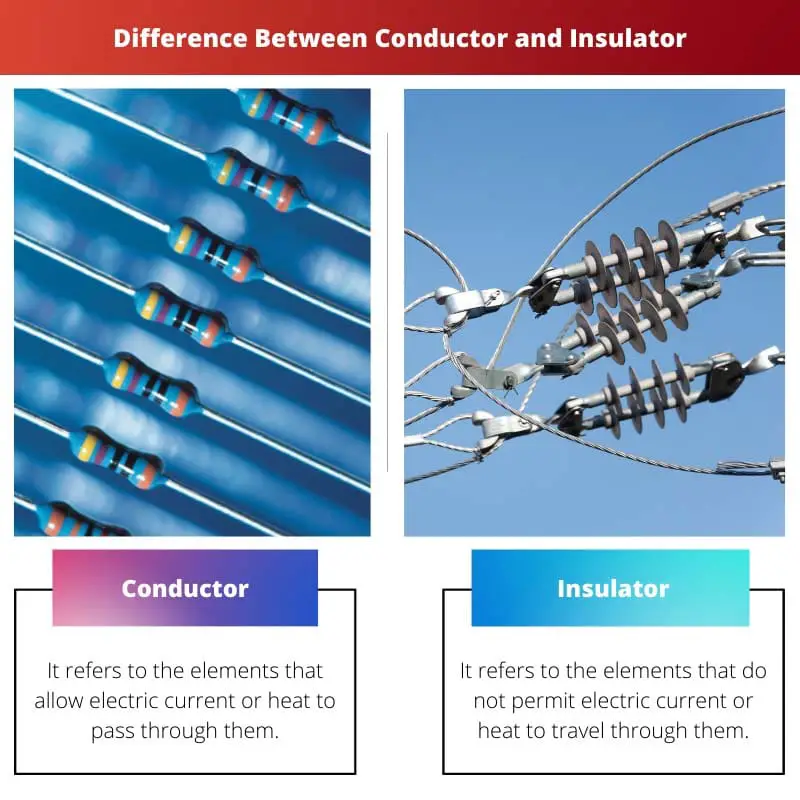
A conductor is a material or an object that allows the flow of electrons freely through it, which makes it useful for carrying electric current. An insulator is a material or an object that resists the flow of electrons, hence stopping electric current from passing through it.

A conductor is described as a material that permits electrons to flow freely and easily from one particular to another in one or more than one direction.
Such free flow of electrons allows heat or electric charge energy to pass through the material quickly.
On the other hand, an Insulator is a material that does not permit electrons to flow freely.
On the contrary, it holds the electrons tightly within the atoms of a material. Consequently, it obstructs the free flow of energy from heat or electric current passing through the material.
Comparison Table
| Parameter of Comparison | Conductor | Insulator |
|---|---|---|
| Definition | It refers to the elements that allow electric current or heat to pass through them. | It refers to the elements that do not permit electric current or heat to travel through them. |
| Electrons | It has free-flowing electrons. | It has tightly knit electrons. |
| Electric field | It is found on the surface of the material. | It does not exist in the material. |
| Conductivity | High | Low |
| Used for | Making electric wires, switches and sockets. | Making the outer cover of the wires, switches and sockets. |
What is Conductor?
v They have high conductivity and poor resistance to electric or thermal energy flow.
This happens because of the presence of ‘free electrons’ in the atomic structure of a conductor.
‘Free electrons’ refer to those electrons which can be easily exchanged with the electrons of other atoms. That is, their bond with the atom of which they are a part lacks strength.
This lack of strength permits the free flow of energy from one atom to another.
The extent to which a material or a substance allows charges or heat to travel through it depends on the number of ‘free electrons’ in its atoms’ outermost orbits.
A substance or material can be considered a good conductor if it has more ‘free electrons’ in the outermost or peripheral shells of its atoms.
Also, there should be no space between the conduction band and the valence band (known as the forbidden energy gap) so that the electrons can quickly move to other atoms.
An object that is made of a material which has conducting qualities will receive the charges passed on it from another object and allow those charges to get distributed all over its surface
unless the repulsive forces between the surplus electrons reduce to the maximum extent possible.
Exchanging charges between two objects becomes easy if both contain conducting materials.
Interestingly, most conductors are made up of metals such as mercury, copper, aluminium, silver, and so on.
Among these, silver is considered the best conductor but is not used for making electric wires because its cost is very high.

What is Insulator?
It is described as a substance or material that retards or blocks the flow of electric current or heat. Insulators have low conductivity and high resistance to thermal or electric energy flow.
This happens because the atoms present in the insulators have a powerful covalent bond between them. Consequently, there is no free movement or exchange of electrons.
Also, insulators have a vast space known as the forbidden gap between the conduction band and the valence band, which demands a lot of energy from the valence electrons to pass through this gap and reach the conduction band.
When some amount of charge or heat is passed on to an object made up of an insulating material, it remains at the starting position and does not get dispersed across the outer layer of the thing.
Consequently, one needs to rub that object with a suitable material so that it gets charged. Another method that can be used to charge such an object is induction.
In an electric circuit, insulators are mainly employed to keep the conductors apart from each other and the other objects around the course.
Insulators ensure that the current flowing through the wires remains within the wire and does not move away to any other object made up of a conducting material.
In the case of thermal energy, they break up the heat flow path by absorbing radiant heat. Most insulators comprise non-metals like rubber, plastic, porcelain, mica, fibreglass etc.
Main Differences Between Conductor and Insulator
- A conductor allows energy, e.g. electric charge or heat, to pass through quickly. At the same time, an insulator does not let electric current or heat travel through it.
- Insulators have molecular solid bonds. At the same time, molecular bonds are fragile in conductors.
- Insulators have very low conductivity. While in conductors, it is very high.
- Insulators have a very high resistance, so the electrons are held together very firmly. The conductors, on the other hand, have meagre resistance.
- Insulators do not have any electric field, neither inside nor on the surface. While in conductors, it is found on the surface and continues to be zero in the inner part of the conductor.

While the technical content is quite detailed, it is presented in a way that’s digestible and engaging.
I appreciate the article managing to be informative yet not overly dry or inaccessible in its scientific content.
Agreed, this article strikes a great balance between technical depth and reader accessibility.
I appreciate the use of examples and references to materials in explaining both conductors and insulators.
The breakdown of what makes a material a good conductor is articulated very effectively in this article.
The scientific complexities of electron movement in conductors and insulators are laid out in a manner that’s easy to comprehend.
Absolutely, the deeper insights into why certain materials are conductors and others are insulators are fascinating.
The practical applications of conductors and insulators are well-contextualized with their scientific properties in this article.
Great explanation! I appreciate how the key takeaways are outlined in a concise manner for easy understanding.
Yes, the comparison table is helpful for quickly grasping the key differences between conductors and insulators.
I agree, the differentiation between conductors and insulators is very clear in this article.
The article effectively clarifies the key differences between conductors and insulators, serving as a great educational resource.
Certainly, this piece seems ideal for students or learners looking to understand the nuances of conductivity.
Who knew learning about conductors and insulators could be so enjoyable? Kudos to the author for an engaging read!
Absolutely, the author has succeeded in making a relatively complex topic accessible and enjoyable with this article.
I find the detailed explanations about conductors and insulators to be enlightening.
Absolutely, the information provided about ‘free electrons’ in conductors is quite interesting and adds depth to the understanding.
The article does well to convey the differences and functions of conductors and insulators in a clear and organized manner.
I couldn’t agree more. The various parameters and comparisons are presented logically and helpfully.
I think the examples of materials that are good conductors and insulators further solidify the understanding of these concepts.
Absolutely, the inclusion of the metals like copper and silver as conductors is helpful for practical application.
The mention of the cost factor in using silver as a conductor showcases practical considerations along with theoretical knowledge.
The terminology used in describing ‘free electrons’ and ‘forbidden energy gaps’ is very technical but explained clearly here.
I’m impressed by the depth of detail in explaining how conductors and insulators function at an atomic level.
Definitely, the discussion on the atomic structure and conductivity is comprehensive yet accessible to readers.