In chemistry, there’re a lot of elements that altogether form a periodic system, and every element comes under some periodic table group.
Each chemical group has similar chemical properties, including valence electrons, atomic size, metallic character, and reactivity.
Coming to the halogen group consists of fluorine (F), chlorine(Cl), bromine(Br), iodine(I), and astatine(At). The halogen group exhibits similar nonmetallic chemical properties. Though, each element has its characteristics and properties.
Key Takeaways
- Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas.
- Bromine is less volatile and more stable at high temperatures, making it ideal for hot tubs, while chlorine is more cost-effective and widely used for pools.
- Both elements require regular monitoring and adjustment to maintain optimal water chemistry, but bromine is considered less irritating to the skin and eyes than chlorine.
Bromine vs Chlorine
Bromine is a reddish-brown liquid at room temperature, is the only non-metallic element that is liquid at this temperature, and has an atomic number of 35 and a symbol of Br. Chlorine is a yellow-green gas at room temperature, used in producing PVCs and has an atomic number of 17 and a symbol of Cl.

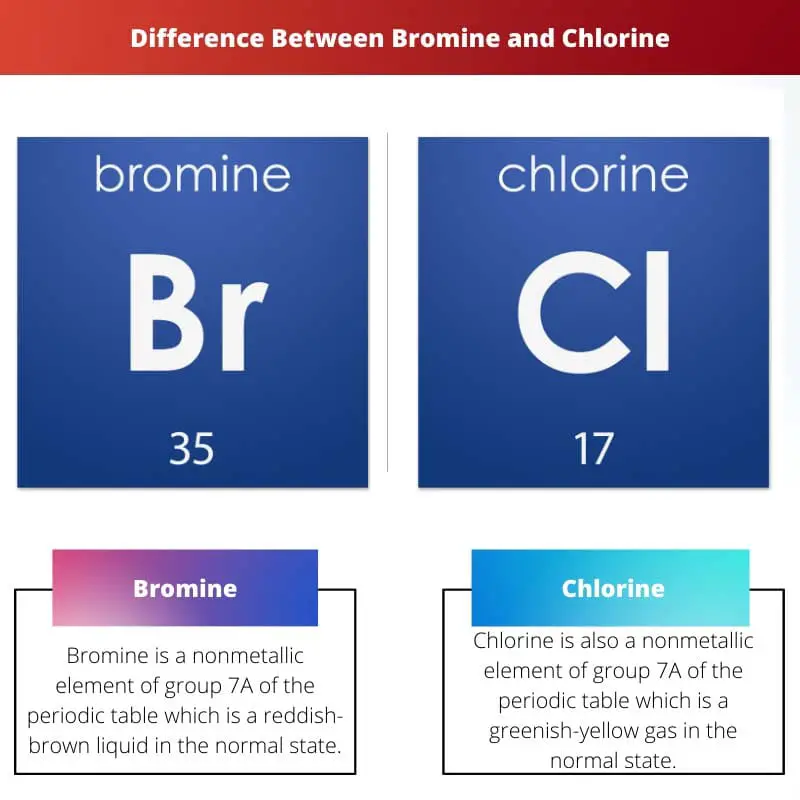
Bromine is a nonmetallic element of group 7A of the periodic table, which is a reddish-brown liquid in the normal state. It has the atomic number 35 with a 79.904 atomic mass. As for the reactivity, bromine is considered less reactive.
However, it does contribute to many interactions. Moreover, it naturally occurs in two stable isotopes but in the form of a mixture of these isotopes. It oxidizes in a -1 configuration, although it can also have a positive oxidation state (+1, +3, +5, +7).
Chlorine is also a nonmetallic element of group 7A of the periodic table, a greenish-yellow gas in the normal state. It has the atomic number 17 with a 35.453 atomic mass.
As for the reactivity, chlorine is considered very reactive. Moreover, it naturally occurs in the form of chemical compounds or disassociated ions. It has all oxidization states from +1 to +7, except +2.
Comparison Table
| Parameters of comparison | Bromine | Chlorine |
|---|---|---|
| State (Under normal conditions) | Bromine is a reddish-brown liquid. | Chlorine is a greenish-yellow gas in a normal state. |
| Atomic number | Bromine is number 35 in the periodic table. | Chlorine is number 17 in the periodic table. |
| Atomic mass | As for the atomic mass, bromine has a 79.904 atomic mass. | Chlorine has an atomic mass of 35.453. |
| Reactivity | Bromine is less reactive, although it does contribute to many chemical interactions. | Chlorine is very reactive under normal conditions. |
| Natura Occurrence | Bromine naturally occurs in two stable isotopes. | Chlorine naturally occurs in a chemical compound or disassociated ions. |
| Oxidation states | Bromine oxidizes in a -1 configuration, although it can also have a positive oxidation state (+1, +3, +5, +7). | Chlorine has all oxidization states from +1 to +7, except +2. |
| Use | Bromine is used in medical preparations, pesticides, dyes, flame retardants, etc. | Chlorine is used in the production of hydrochloric acid, cleaning products, etc. |
What is Bromine?
A periodic table consists of different elements that have different characteristics and properties. A halogen is a group in the periodic table consisting of nonmetallic elements with similar properties in general.
Bromine is a nonmetallic element of group 7A of the periodic table, which is a reddish-brown liquid in the normal state. It has a really strong odour that can be irritating sometimes.
As for the atomic number, it has an atomic number of 35, along with a 79.904 atomic mass.
Unlike chlorine, bromine is less reactive, although it participates in many chemical interactions. Thus, it is considered much more stable than chlorine.
The natural occurrence of bromine is quite stable as it occurs in a mixture of two stable isotopes. Also, it can also be extracted as an impurity in the chlorine mineral halite. Sylvite, Carnallite, etc.
Moreover, the bromine water is composed of an aqueous solution of 2.8% of bromine and is used in many organic reactions. Even the bromine is in a 1:660 ratio in the seawater with the majority of chlorine atoms.
Hence, it has an oxidization state of -1, although it can also have a positive odd oxidation state (+1, +3, +5, +7).
Lastly, bromine can be used in medical preparations, pesticides, dyes, flame retardants, etc.

What is Chlorine?
As mentioned, A periodic table consists of different elements with different characteristics and properties. A halogen is a group in the periodic table consisting of nonmetallic elements with similar properties.
Chlorine is also a nonmetallic element of group 7A of the periodic table, a greenish-yellow gas in the normal state. It has a very strong odour that can be irritating. As for the atomic number, it has an atomic number of 17, along with a 35.453 atomic mass.
Unlike bromine, chlorine is very reactive, therefore, it reacts more and involves a variety of chemical interactions.
The natural occurrence of chlorine is in the form of a chemical compound or disassociated forms of ions.
Also, it can be obtained from several minerals, such as halite, sylvite, carnalite, etc. Additionally, there are anions of chlorine present in the seas and oceans. Apparently, the bromine is in a 1:660 ratio in the seawater with the majority of chlorine atoms.
Besides, chlorine is a strong oxidant and with an -1 oxidation state. Although chlorine has a positive oxidation state when there’s a stronger oxidant present in the molecule. It has all oxidization states from +1 to +7, except +2.
Lastly, Chlorine is used in the production of hydrochloric acid, cleaning products, etc.

Main Differences Between Bromine and Chlorine
Bromine and Chlorine belong to the same halogen group in the periodic table and are nonmetallic elements. Due to their similar properties and characterization, they belong to the same group in the periodic table.
People tend to see them in the same light, but they are very different from each other. Yet, they have common properties and characterization as well.
- Bromine is a reddish-brown liquid. Meanwhile, chlorine is a greenish-yellow gas in a normal state.
- Bromine is number 35 in the periodic table, while chlorine is number 17 in the periodic table.
- As for the atomic mass, bromine has a 79.904 atomic mass. Meanwhile, chlorine has an atomic mass of 35.453.
- Bromine is less reactive, although it does contribute to many chemical interactions, while chlorine is very reactive under normal conditions.
- Bromine naturally occurs in two stable isotopes, while chlorine naturally occurs in a chemical compound or disassociated ions.
- Bromine oxidizes in a -1 configuration, although it can also have a positive oxidation state (+1, +3, +5, +7). Meanwhile, chlorine has all oxidization states from +1 to +7, except +2.
- https://www.sciencedirect.com/science/article/pii/S0043135400002165
- https://pubs.acs.org/doi/pdf/10.1021/ic00103a040

The article provides an in-depth analysis of bromine and chlorine, offering valuable insights into their reactivity, occurrence, and uses. The comparison table is particularly helpful in summarizing the key differences between the two elements.
This article is a comprehensive resource for anyone seeking to expand their knowledge of chemistry. The detailed descriptions of bromine and chlorine, along with their individual characteristics, make for an informative read.
This article effectively captures the essence of bromine and chlorine, providing an engaging narrative on the distinctive properties and applications of these elements. It is a testament to the author’s expertise in the field of chemistry.
The thorough exploration of bromine and chlorine elucidates the significance of these elements in various industries. The article serves as a valuable reference for chemists, environmental scientists, and researchers seeking a deeper understanding of halogens.
The detailed comparison between bromine and chlorine is truly enlightening. Having a clear understanding of their characteristics and properties can be beneficial in various practical applications.
While the information presented is quite detailed, it could benefit from a more critical analysis of the potential environmental impact of bromine and chlorine, particularly in the context of their widespread use in disinfectants.
It is always fascinating to study the properties of different elements, such as bromine and chlorine. The information provided is very useful for science students and anyone interested in chemistry.
The comparison of bromine and chlorine is indeed intriguing. However, it would be interesting to explore the potential applications of these elements beyond the scope of their conventional uses, shedding light on emerging trends and technologies.