The prefix hypo denotes “inadequate.” Solute concentrations in a hypotonic solution are less than in cells. Hyper signifies “too much.”
A hypertonic solution contains more solutes than that of the cells and has a greater pressure outside than inside. And from word iso, it signifies that isotonic mixtures should retain their regular form when subjected to solutions.
Key Takeaways
- Hypertonic solutions have higher solute concentrations than the cell’s interior, causing water to leave and shrink.
- Hypotonic solutions have lower solute concentrations, leading to water entering the cell, making it swell or even burst.
- Isotonic solutions have equal solute concentrations inside and outside the cell, maintaining their size and shape due to equal water movement.
Hypertonic vs Hypotonic vs Isotonic
Hypertonic means a solution with more solutes than its cells and more external pressure. Hypotonic is used to describe a solution with fewer solute concentrations than the cells. Isotonic means any solution with equal solute concentrations and cells; it is also equal to external solutions like body fluids.
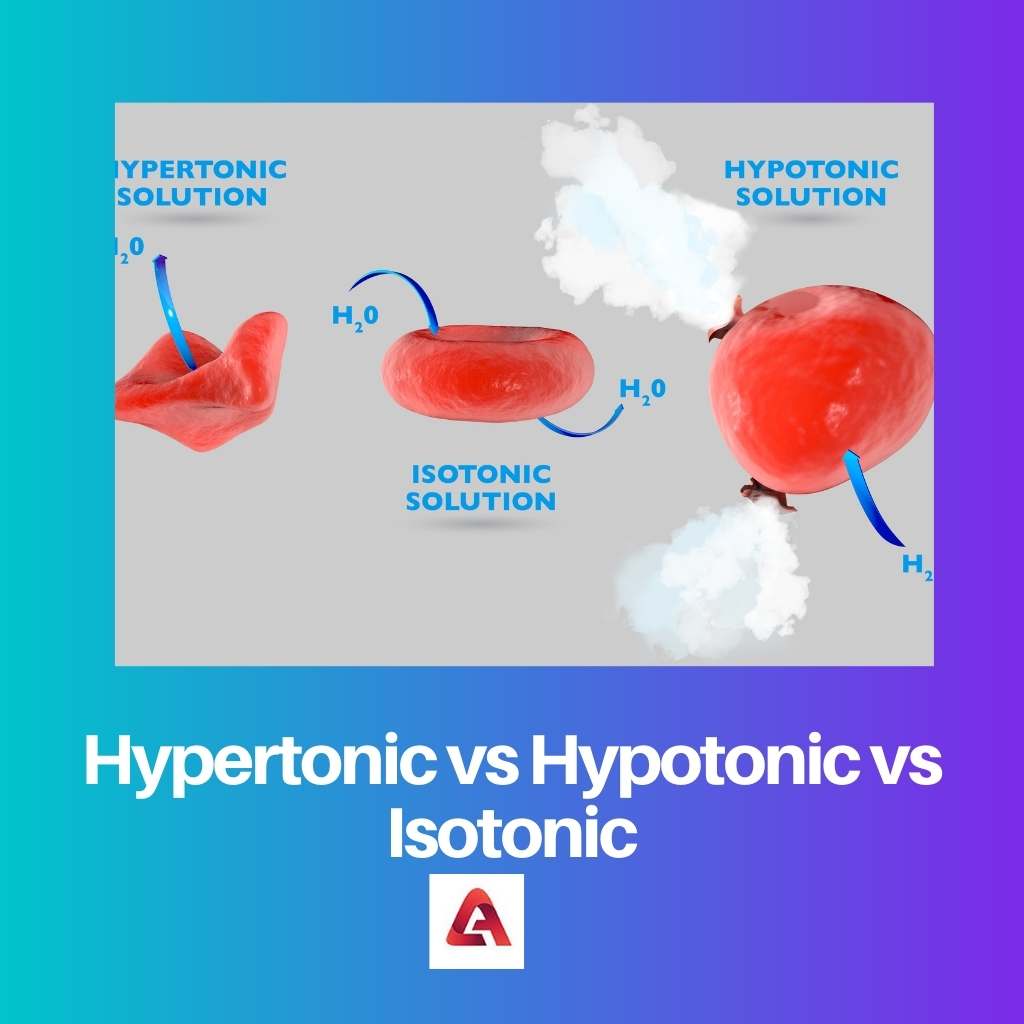
When the external solution includes a larger concentration of particles, and the internal solutions of the cells include a lower density, the system is hypertonic.
Since it seeks to dilute the outer solution, fluid is forced to escape the cell and enter the outside. This dilution results in a reduced quantity from the outside that is nearer to the level from the inside.
A hypotonic mechanism as opposed to a hypertonic system. A hypotonic system seems to have a concentration of solutes from the inside of the cell than on the outside, which has a lower density.
This powers water into the cell, diluting the inside. Again, the goal is to achieve equilibrium by bringing the concentration levels to something like a level comparable.
Osmosis and tonicity are concerned with achieving a balance of concentrations here between the interior and exterior of such membranes. When that equilibrium is reached, the system is indeed not isotonic.
Comparison Table
| Parameters of Comparison | Hypertonic | Hypotonic | Isotonic |
|---|---|---|---|
| Meaning | In Hypertonic configuration, the fluids just outside of the cell have a higher concentration of solutes than the fluids within the cell. | The fluids just outside of the cell have a lower solute concentration than the fluids within the cell throughout hypotonic configuration. | Isotonic solutions comprise equal quantities of solutes. |
| Role as Preservative | Hypertonic Solutions are quite effective against food preservation. | Hypotonic solutions remain ineffective for preservation. | Isotonic solutions are ineffective for preserving food. |
| Osmotic Pressure | Hypertonic fluids have a greater osmotic pressure than other liquids. | Hypotonic solutions are those with regions of lower pressures. | Isotonic solutions have the very same osmotic pressure. |
| Effect on cells | Cells shrink when exposed to hypertonic solutions. | Cells inflate under hypotonic conditions. | Cells are unaffected by isotonic solutions. |
| Dissolving capacity | Hypertonic solutions have fixation with a Lower Dissolvable capacity. | In the situation of hypotonic solutions, there’s also a high soluble fixation. | In case of isotonic solutions, the dissolvable fixation is equal and decent enough. |
What is Hypertonic?
When the external solution includes a larger concentration of particles, and the internal solutions of the cells include a lower density, the system is hypertonic.
Since it seeks to dilute the outer solution, fluid is forced to escape the cell and enter the outside. This dilution results in a reduced quantity from the outside that is nearer to the level from the inside.
The flow of the fluid from cells to the exterior causes the cell to shrivel. When so much water is taken from RBCs, they will contract and then become deformed. This structural breakdown reduces the red blood cell’s ability to operate.
Plants wither and thus become twisted if they are not watered since water is migrating out from the cells, creating a decrease in turgor pressure, which seems to be the additional pressure used by the plants to shove against the cell membrane and retain its shape.

What is Hypotonic?
A hypotonic mechanism as opposed to a hypertonic system. A hypotonic system seems to have a concentration of solutes from the inside of the cell than on the outside, which has a lower density.
This powers water into the cell, diluting the inside. Again, the goal is to achieve equilibrium by bringing the concentration levels to something like a level comparable.
As water enters the cells, it raises internal tensions and leads the cells to swell. These cells can explode if they grow excessively large. This can also be beneficial to some microbes.
Hypertonicity is a negative idea because it lowers turgidity in plants. However, hypotonicity causes greater turgor pressure, which is beneficial to immature plants.
Plants stimulate mechanisms that raise turgor pressure since it presses upon cell walls and allows plant cells to expand so that they might keep growing.
What is Isotonic?
Osmosis and tonicity are concerned with achieving a balance of concentrations here between the interior and exterior of such membranes. When that equilibrium is reached, the system is indeed not isotonic.
Water enters the cell at the very same pace that it exits, resulting in a nearly zero flow of water. This equilibrium generates a stable shape for the cellular and is the topmost priority for the majority of biological cells.
To avoid function loss, our RBCs favour this condition over another two.
Since these things happen in living organisms, they are comparative words that continually move and alter as humans eat or do not ingest fluids, permeability solutes (such salts) flow to and from cells, and any other factor that modifies these levels.
Plant cells, unlike human cells, prefer to be hypotonic instead of isotonic since it boosts turgidity and retains the cells in a much stiff and stronger structure.

Main Differences Between Hypertonic, Hypotonic, and Isotonic
- In the Hypertonic configuration, the fluids just outside the cell have a higher concentration of solutes than those within the cell. The fluids just outside the cell have a lower solute concentration than those within the cell throughout the hypotonic configuration. Isotonic solutions comprise equal quantities of solutes.
- Hypertonic Solutions are quite effective against food preservation. Hypotonic solutions remain ineffective for preservation. Isotonic solutions are ineffective for preserving food.
- Hypertonic fluids have a greater osmotic pressure than other liquids. Hypotonic solutions are those with regions of lower pressures. Isotonic solutions have the very same osmotic pressure.
- Cells shrink when exposed to hypertonic solutions. Cells inflate under hypotonic conditions. Cells are unaffected by isotonic solutions.
- Hypertonic solutions have a fixation with a Lower Dissolvable capacity. In the situation of hypotonic solutions, there’s also a high soluble fixation. In the case of isotonic solutions, the dissolvable fixation is equal and decent enough.
- https://adc.bmj.com/content/91/10/828.short
- https://web.p.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=11749210&AN=91578481&h=B8Qa%2btAy8MllQjCeC4Wpa1x3PgR0TNKJ9e0Q1jNj0NtGaxvh0ekgjofZcWRsn6C2K0EfMTMa4Zu3Phq0%2bLArTA%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d11749210%26AN%3d91578481
Last Updated : 11 June, 2023

Piyush Yadav has spent the past 25 years working as a physicist in the local community. He is a physicist passionate about making science more accessible to our readers. He holds a BSc in Natural Sciences and Post Graduate Diploma in Environmental Science. You can read more about him on his bio page.

Thank you for explaining the concept of hypertonic, hypotonic, and isotonic solutions with such clarity.
The explanation of hypertonic, hypotonic, and isotonic solutions is very thorough and easy to understand
I agree, the examples provided make it easier to grasp the concepts.
This article provides a comprehensive understanding of these tonicity concepts.
This is an excellent piece of information, I had no idea about some of these concepts
Agreed, very detailed and well-explained.
The clarity of the definitions and comparison of hypertonic, hypotonic, and isotonic solutions is commendable.
This article is a great resource for understanding the differences between hypertonic, hypotonic, and isotonic solutions.
Thank you for the detailed explanation, it was very informative.
Great article, it has enhanced my knowledge on tonicity and its implications.
This article explains the impact of hypertonic, hypotonic, and isotonic solutions on cells very effectively
I completely agree, the differentiation is very well-explained.