Thermodynamics is a field of chemistry that deals with the work done and heat. Particularly the relationship between the two.
The relation is established during ongoing chemical reactions or when any change. It is seen in the physical state of the reactants and products.
It is not only confined to the practical calculations of the reactions but also comprises the mathematical relations and calculations related to it.
Key Takeaways
- An isotonic solution has the same concentration of solutes as inside the cell, while Equilibrium refers to a state of balance.
- Isotonic solutions help maintain cell shape and size, while Equilibrium helps maintain a balance between reactants and products in a chemical reaction.
- An example of an isotonic solution is a saline solution, while an example of Equilibrium is when CO2 and H2O react to form carbonic acid.
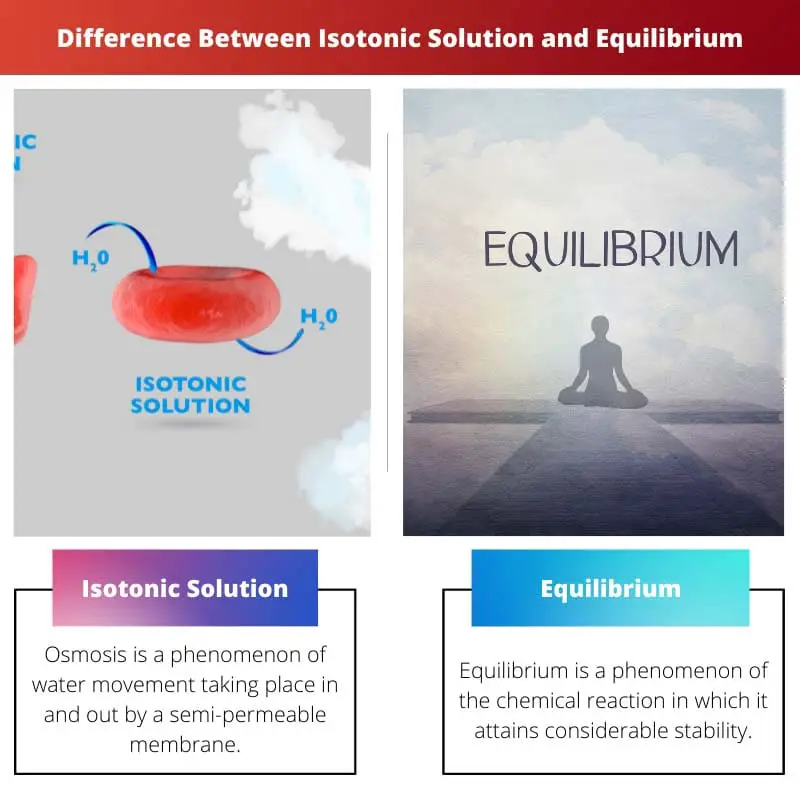
Isotonic Solution vs Equilibrium
Isotonic solution is a solution where the concentration gradient is equal to the solute and solvent passing by a semi-permeable membrane. The movement of the solute and solvent is equal. Equilibrium is an occurrence of a chemical reaction when it attains stability and when forward, and reverse reactions are balanced.

Isotonic Solution is one of the three types of Solution known apart for this. Hypertonic Solution and hypotonic Solution is known.
The solution can acquire an isotonic condition when both the constituent, that is, solute & solvent, persists in the same concentration. A good example of such a condition is the blood cells of the human body.
They allow the nutrients, water, and other materials to pass through their membrane to carry oxygen mainly. Equilibrium is a condition in the chemical solution when both the reactants and products.
Those are stable or at an equal rate. In other words, the forward and reverse reaction occurs such that the resultant product is obtained, which again breakdown in the reactants.
For a respective reaction, the reaction rate of the forward one and for the reverse one might be the same but never tends to be zero in equilibrium.
Comparison Table
| Parameters of Comparison | Isotonic Solution | Equilibrium |
|---|---|---|
| Discovered By | Sydney Ringer | Gibbs and Le Chatelier |
| Year | 1882 | 1873 |
| Definition | The concentration of constituent (solute and solvent) passing by the semi-permeable membrane is the same. | When forward and reverse reactions are balanced or stable |
| Preparation | It can be manually prepared | It cannot be prepared manually as it attains it by itself |
| Physical Parameters | No such effect | Affected by the rate of reaction, temperature, pressure, etc |
| Movement | No movement is shown because of the same concentration gradient | No movement because of zero net force |
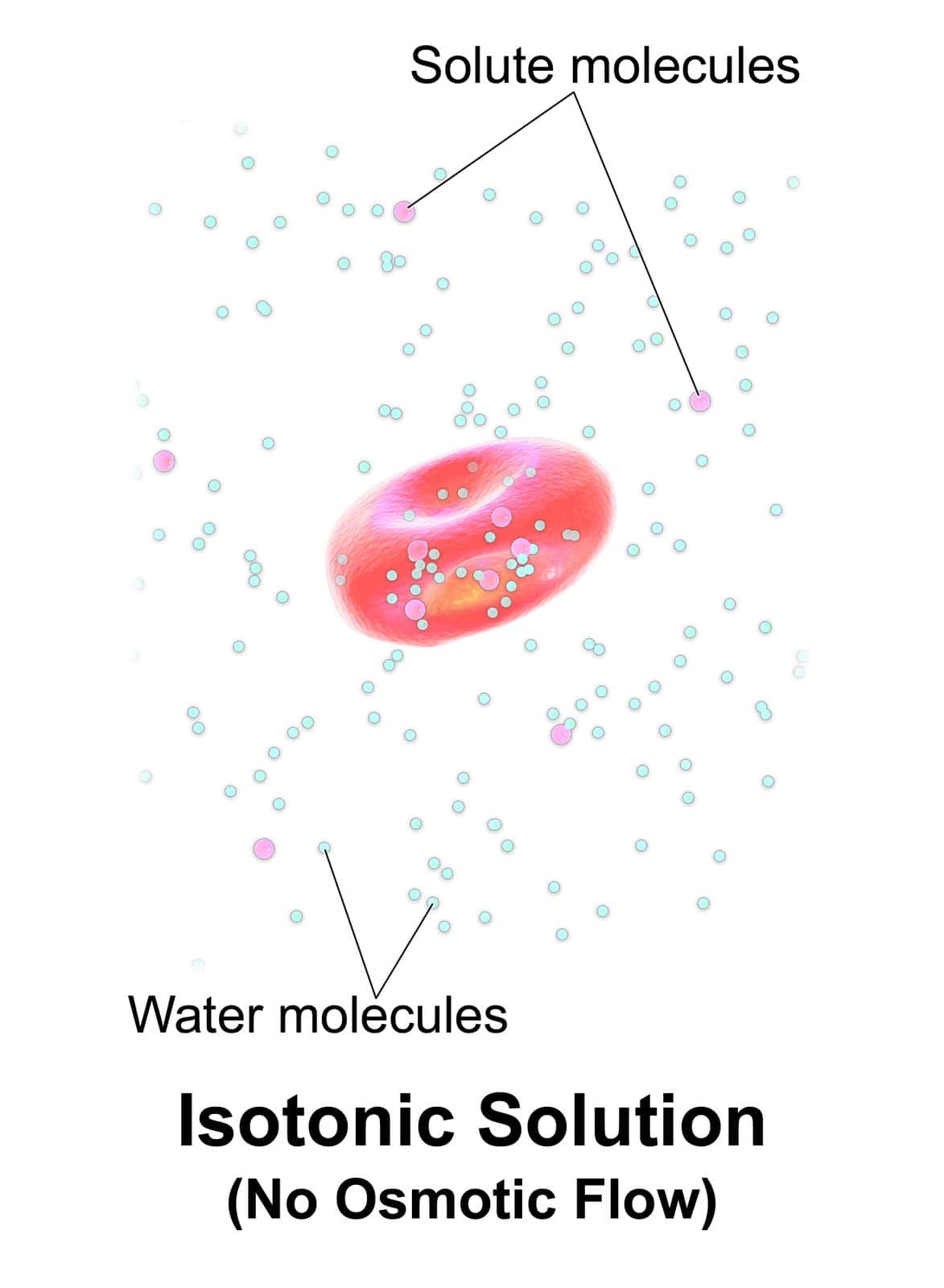
What is Isotonic Solution?
Osmosis is a phenomenon of water movement taking place in and out by a semi-permeable membrane. This is further subdivided into three types of Solutions that is – Hypotonic Solution.
Hypertonic Solutions and Isotonic Solutions. Isotonic Solution is where the concentration gradient of the Solution is the same (of the solute and solvent) passing by the semi-permeable membrane.
The word itself means equal, and thus the movement of both solute and solvent is equal. Sydney Ringer discovered the phenomenon of the isotonic Solution in the year 1882.
The phenomenon of isotonic Solution can be established in the laboratory by preparing it manually. The isotonic solution is said to be not affected by any external factors.
Such as – the rate of reaction, pressure, temperature, etc. Also, the movement of particles is so slow in the Solution that it isn’t even noticeable.
What is Equilibrium?
Equilibrium is a phenomenon of the chemical reaction in which it attains considerable stability. In other words, when the two reactions are forward in which, the reactants change.
Into product and the backward reaction in which the products again break down. To form reactants, attain stability at a considerable point where the reactants can be further broken.
And the product doesn’t break back to form reactants. The word equilibrium itself means equal and stability.
Besides this, the phenomenon of equilibrium was discovered by Gibbs and Le Chatelier. They both worked upon it in a different timeline that is Gibbs worked from 1873-1878.
While Le Chatelier worked in 1875, many other scientists also worked on this discovery.
Perhaps the phenomenon is affected by external factors like – reaction rate, temperature, pressure, etc. And the movement is unnoticed because of the forces working on it that are not equal to zero.
Main Differences Between Isotonic Solution and Equilibrium
- The phenomenon of Isotonic Solution was discovered by the scientist Sydney Ringer whereas comparatively, on other hand, the phenomenon of Equilibrium was discovered by the two scientists Gibbs and Le Chatelier.
- The discovery of the isotonic solution phenomenon was made in the year 1882, whereas comparatively, on the other hand, the discovery of the equilibrium phenomenon was made first in the year 1873, and then later, different scientists worked on it on a different timeline.
- The phenomenon of the isotonic Solution can be stated as to when the concentration gradients of a solution (both solute and solvent) passing by the semi-permeable membrane are the same, whereas comparatively, on the other hand, the phenomenon of the equilibrium can be stated as the forward and backward reaction of the Solutions are stable.
- The Solution of defining isotonic Solution can be manually prepared, whereas comparatively, on the other hand, the equilibrium reactions can not be manually prepared as they attain it themselves at a particular point.
- The isotonic Solution eventually doesn’t get affected by the external physical factors, whereas comparatively, on the other hand, the equilibrium phenomenon tends to be affected by the external phenomenon such as – temperature, pressure, concentration, reaction rate, etc.
- In an isotonic solution, the movement of constituents by the semi-permeable membrane is very slow, and it thus occurs by the process of osmosis, whereas comparatively, on the other hand, in the equilibrium, the movement isn’t noticeable because of the equal net force that is zero.
References
- https://link.springer.com/article/10.1007/BF02989804
- https://pubs.acs.org/doi/pdf/10.1021/je60058a011
- https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2818.1985.tb02641.x
- https://wires.onlinelibrary.wiley.com/doi/abs/10.1002/wcs.108
Last Updated : 11 June, 2023

Piyush Yadav has spent the past 25 years working as a physicist in the local community. He is a physicist passionate about making science more accessible to our readers. He holds a BSc in Natural Sciences and Post Graduate Diploma in Environmental Science. You can read more about him on his bio page.

The detailed comparison between isotonic solutions and equilibrium and the explanation of their main differences is very educational. It’s interesting to learn that isotonic solutions can be manually prepared, while equilibrium reactions occur naturally.
The information provided about osmosis and the three types of solutions, including isotonic solutions, is very elucidating. It’s fascinating to learn how isotonic solutions are not affected by external physical factors.
This article provides a clear explanation of the concepts of isotonic solution and equilibrium. The distinction between the movement of solute and solvent in an isotonic solution and the balancing of forward and reverse reactions in equilibrium is well-explained.
I agree, the article effectively highlights the differences between isotonic solutions and equilibrium with well-detailed information.
The detailed explanation of the discovery and definition of isotonic solutions and equilibrium is enlightening. The assertion that the isotonic solution doesn’t get affected by external physical factors is intriguing.
Thank you for this comprehensive explanation of isotonic solutions and chemical equilibrium. It is clear that the movement of solute and solvent is equal in isotonic solutions, while equilibrium refers to balancing forward and reverse reactions.
The detailed explanation of isotonic solutions and equilibrium, along with the comparison table, offers a comprehensive understanding of these concepts. I appreciate the clear explanation of the difference in movement between isotonic solutions and equilibrium reactions.
The comparison table is very helpful in understanding the main differences between isotonic solutions and equilibrium. It is interesting to note that isotonic solutions can be manually prepared, while equilibrium reactions occur naturally.
The explanation of isotonic solutions and equilibrium, along with their definitions and main differences, is thorough and informative. The examples used to illustrate isotonic solutions and equilibrium are also very helpful.