When a lot of heat is put into a solid state, It changes into its liquid state and then into its gaseous if more heat is provided. But some substances directly transform from their solid state to their gaseous state.
However, when a substance changes its phase, there are various factors like temperature and pressure that causes this transition. Sublimation and deposition are two phases.
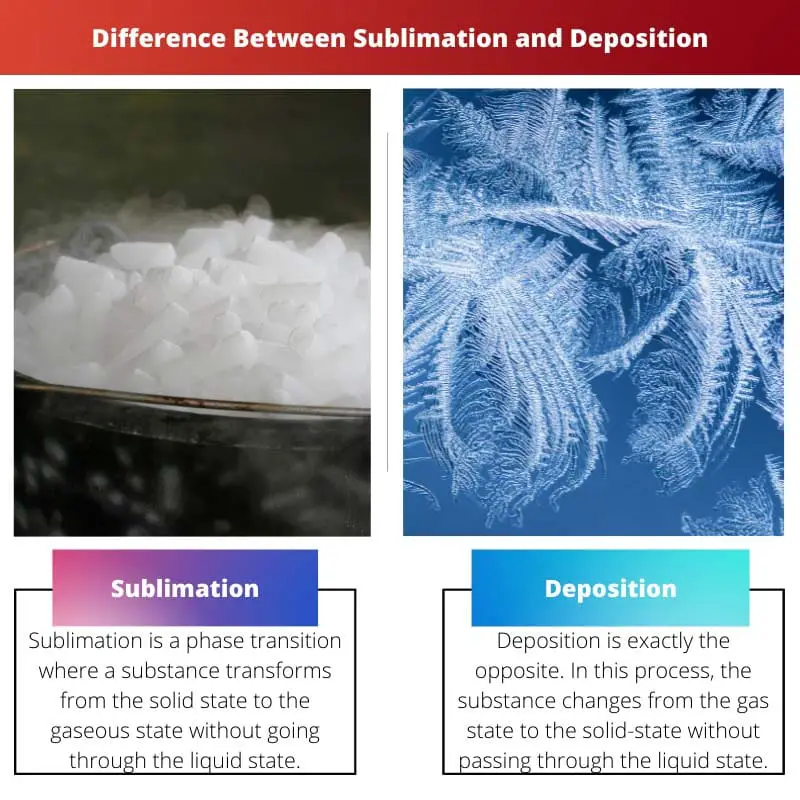
Sublimation is a phase where a substance directly switches from the solid to the gas state, whereas in a deposition, the substance changes into the gaseous state without passing through the liquid state.
Key Takeaways
- Sublimation is the process of a solid changing directly into a gas without going through the liquid phase.
- A deposition is the process of a gas changing directly into a solid without going through the liquid phase.
- Sublimation occurs with an increase in temperature and a decrease in pressure, while deposition occurs with a decrease in temperature and an increase in pressure.
Sublimation vs Deposition
Sublimation is a term used to describe the process in which a solid substance directly changes into a gas state without passing through the liquid state. Deposition is a term used to describe the process in which a gas changes directly into a solid without first becoming a liquid.

Sublimation is a phase where a substance is changed into a gaseous state from its solid form without experiencing the liquid state. Though this process consumes a lot of heat, it does not alter the chemical combinations of that substance.
On the other hand, deposition is exactly the opposite of sublimation. In the deposition, the substance changes from the gas state to the solid state without passing through the liquid phase. This process releases a lot of energy, unlike sublimation.
However, both of these processes do not include a liquid phase.
Comparison Table
| Parameters of Comparison | Sublimation | Deposition |
|---|---|---|
| Definition | Sublimation is a phase transition where a substance transforms from the solid state to the gaseous state without going through the liquid state. | On the other hand, deposition is exactly the opposite. In this process, the substance changes from the gas state to the solid state without passing through the liquid state. |
| Purpose | Sublimation is used to break chemical compounds of substances to achieve their purified state. | This process is used to make ice from evaporated water. |
| Consumption of energy | Sublimation consumes a lot of energy in the process, but the chemical compounds of the substance remain intact. | Unlike sublimation, deposition releases a lot of energy. |
| Process | In sublimation, a substance transforms into a gaseous state from its solid state. | But in deposition, the process is reversed. The substance is not solidified but changes into its gaseous form. |
| Heat | Since this process releases a lot of heat, it is called an endothermic process. | In this process, a lot of heat is released. Thus, it is called an exothermic process. |
What is Sublimation?
Sublimation is a type of phase transition where a solid substance changes its form and becomes gas without passing through the liquid state. It is an endothermic process.
An endothermic reaction is a chemical reaction requiring more energy to consume heat. A constant input of energy in the form of heat is needed to keep an endothermic reaction going.
Dry ice is an example of sublimation. Dry ice is solid carbon dioxide. It can be seen that when carbon dioxide is exposed to room temperature, it will go straight from its solid state to gas.
Camphor, Naphthalene, Ammonium Chloride, and Anthracene are examples of such substances that do not pass through a liquid state. Sublimation is the process of converting the substance from its solid state to its gaseous form.
This process can be used to separate a few components of a chemical mixture. A mixture of ammonium chloride and salt is taken in a china dish, and an inverted fannel is positioned on top of that. The fannel should be closed with something like cotton.
Then heat would be given from a burner to the mixture. As a result, ammonium chloride will start releasing vapour, but the salt will not. And the vaporized substance would be solidified.
What is Deposition?
A deposition is also a phase change where an object changes from its gaseous state to its solid form without passing through the liquid state. It is a reversed process of sublimation. So it is called de-sublimation.
It is an exothermic process as this reaction releases a lot of energy or heat. When a gas is cooled down, it converts into liquid and then into a solid state. But in this method, the substance does not change into a liquid state.
But a few objects directly convert into their solid state from their gaseous form. As observed in the sublimation process, solid ammonium chloride has been transformed into fumes without entering its liquid form.
When the heat of the burner is turned off, it can be seen that the vaporized ammonium chloride returns to its solid form without changing itself into liquid.
Main Differences Between Sublimation and Deposition
- In sublimation, an object changes from its solid state to its gas form. In the deposition, a gaseous object is transformed into its solid state.
- Sublimation consumes a lot of energy as heat is needed on a large scale. But in deposition, the rate of releasing heat is high.
- Sublimation is an endothermic process, whereas deposition is an exothermic process.
- In sublimation, gas cannot be changed into a solid object. Similarly, a solid substance cannot be altered into a gas in deposition.
- The method of sublimation is utilized to separate the chemical components of an unpurified compound. On the other hand, deposition is needed to make ice.
- https://www.tandfonline.com/doi/pdf/10.1080/00797308.1955.11822547
- http://althea.ch/wp-content/uploads/2019/08/Sublimation-%E2%80%93-Inquiries-into-Theoretical-Psychoanalysis.pdf
The comparison and contrast between sublimation and deposition are essential in understanding these phase changes. The factors that influence these processes warrant further exploration.
The direct transformation from solid to gas in sublimation and the reverse process in deposition underscore the complexity and significance of these phase changes in different materials.
Sublimation and deposition are two equally interesting and crucial phases that occur and they are essential in various fields. The differences between the two as the amount of energy involved set them apart.
The differences in the energy requirements and the processes involved in sublimation and deposition indicate the significance of understanding these phase transitions in different substances.
The understanding of sublimation and deposition is complex, but it holds great significance in various fields, particularly in chemistry and material sciences.
Sublimation is used to purify substances and separate chemical compounds, and the process is a key area of study in applied chemistry and related fields.
Sublimation is a fascinating process that occurs in various chemical compounds and is crucial in separating mixtures. The energy required, and the ability to purify substances is of great importance.
The process of direct transformation from solid to gas without passing through liquid phase in sublimation is intriguing. The utilization of this process to separate substances is indeed noteworthy.
Deposition, on the other hand, is an exothermic process where a substance changes directly from its gas state to its solid form without passing through the liquid phase. The involved energy release makes it a significant area of study.
The phase changes between sublimation and deposition are influenced by factors like temperature and pressure, and their application in separating chemical components is a crucial area of study.