The use of chemical compounds is done for good as well as bad purposes. In the latter implication, the formation of bombs has become the most threatening business for mankind at large.
There are various types of bombs and they are better than the previous versions in terms of intensity and pricing.
Key Takeaways
- Atomic bombs rely on nuclear fission, the splitting of heavy atoms, to release energy, whereas hydrogen bombs use nuclear fusion, combining light atoms.
- Hydrogen bombs produce significantly more energy and have greater destructive power than atomic bombs.
- The development and use of atomic bombs led to the creation of hydrogen bombs as nations sought more powerful weapons in the nuclear arms race.
Atomic Bomb vs Hydrogen Bomb
An atomic bomb is a nuclear weapon that relies on fission, a reaction in which a nucleus or an atom breaks into two pieces. The hydrogen bomb is a nuclear weapon that relies on fusion, the process of putting two separate atoms together to form a third atom. A hydrogen bomb causes a bigger explosion.

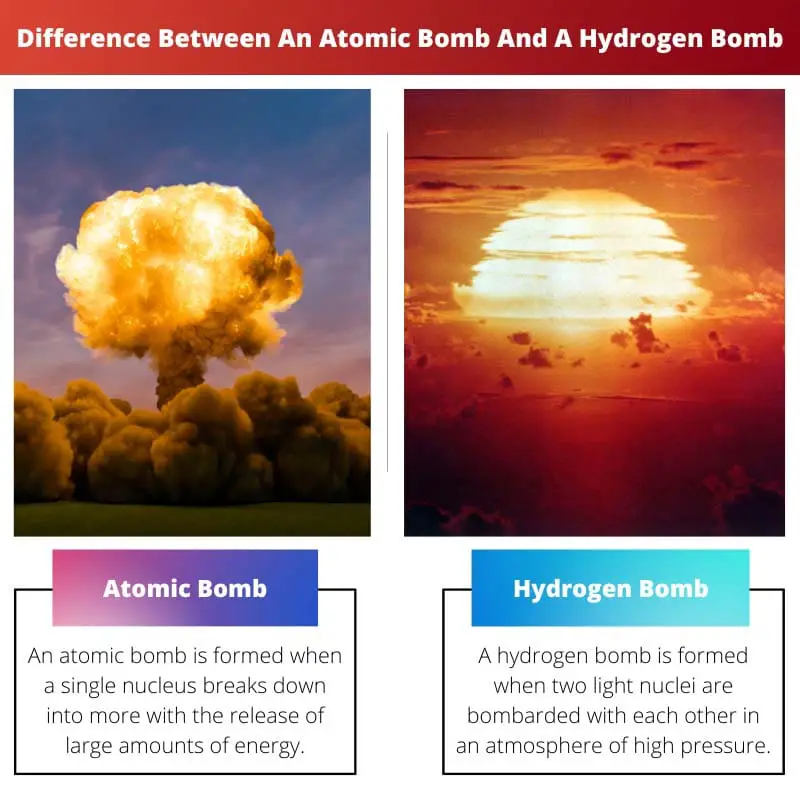
An atomic bomb is formed when a single nucleus breaks down into more with the release of large amounts of energy. The nuclei put to use are extracted from highly powerful radioactive elements that can be sustained for a long time.
‘Little Boy’ was the name of the first atomic bomb.
A hydrogen bomb is formed when two light nuclei are bombarded with each other in an atmosphere of high pressure. No hydrogen bomb has been used in nuclear warfare as of now.
In most countries, successful testing has been conducted. This bomb is an exaggerated version of the atomic bomb.
Comparison Table
| Parameters of Comparison | Atomic Bomb | Hydrogen Bomb |
|---|---|---|
| Nuclear Reaction Used | The atomic bomb uses the principles of the atomic fission reaction. | Hydrogen Bomb works on the lines of nuclear fusion reaction. |
| Products Formed | Krypton and barium nuclei are formed as splitting products. | A larger nucleus of helium is formed in this reaction. |
| Energy Released | Atomic Bomb released 200 MeV of energy per fission. | A hydrogen bomb releases energy equivalent to one thousand times that of an atomic bomb. |
| First Testing | The first atomic bomb was tested in 1945. | The first H-bomb was tested in 1952. |
| Nuclei Used | Atomic Bomb uses plutonium and uranium nuclei. | Hydrogen Bomb uses only the isotopes of hydrogen and a single proton. |
What is Atomic Bomb?
An atomic bomb is also known as a nuclear bomb, owing to the part of the atom used in the construction of the bomb. As per chemical analytics, a fission reaction results in the formation of numerous small nuclei.
This results in mass devastation and the human DNA is also affected due to a series of mutations caused by the harmful particles. The main principle underlying this form of an explosion is the breakdown of a large nucleus into smaller ones leading to the release of splitting energy.
Atomic Bomb mainly uses nuclei of plutonium or uranium 235. These are preferred due to the easy availability and appropriate atomic characteristics.
Further, the splitting of nuclei leads to the formation of Barium and Krypton nuclei and the series of reactions becomes infinite. It also serves as a detonator in a hydrogen bomb since the reaction cannot sustain itself without an existing force.
The atomic bomb, as a thermonuclear weapon, played a crucial role during the second world war. The first country in the world to experience its horrors was Japan (particularly, the cities of Hiroshima and Nagasaki).
The energy released during one blast is equivalent to a TNT explosion amounting to twenty thousand tonnes on average.

What is Hydrogen Bomb?
Hydrogen Bomb works on the principle of nuclear fusion. In this reaction, two nuclei are brought together under an atmosphere of high pressure.
This is done to accelerate the overall fusion process. In all atmospheric conditions, the devastation caused by a hydrogen bomb is higher than that caused by a normal atomic bomb.
Overall, the intensity is based on the size of the bomb and the detonator.
Hydrogen Bombs produce large amounts of energy in the form of heat and light. The mass-energy equivalence of Einstein is put to use while calculating the amount of energy.
This results from the formation of a big nucleus that is the product of the fusion reaction. No external sources are required once the required point is ignited carefully.
After the explosion, a mushroom cloud is formed around the site, and hydrogen nuclei even penetrate the surface of the earth.
Hydrogen Bomb is also believed to follow the fusion reaction that takes place in the stars and sun. The main isotopes of hydrogen used in the incorporation of an H-bomb include deuterium and tritium.
The reason for usage is the light nature which poses no threat to the fusion reaction and has self-catalyzing properties.

Main Differences Between An Atomic Bomb And A Hydrogen Bomb
- Atomic Bomb is formed with the help of nuclear fission reactions that also play a role in electricity generation. On the other hand, hydrogen bomb is formed by nuclear fusion, similar to the energy maintenance in celestial bodies.
- Energy is produced as a common product but atomic bomb gives extra nuclei of Krypton on Barium while hydrogen bomb reduces the reactants to a single Helium nuclei.
- The energy released in atomic bomb is fixed at 200 mega electron volt in every fission that takes place while hydrogen bombs release energy in a proportional manner (thousand times that of energy released by atomic bomb detonator used).
- Atomic bomb was first tested in 1945 while hydrogen bomb came about in 1952.
- Radioactive elements (uranium and plutonium in most cases)are used in atomic bomb while hydrogen bomb uses simplistic hydrogen isotopes only.

The consequences of atomic and hydrogen bombs are truly scary. Their impact is beyond words.
I am both in awe and fear of the immense energy released by these destructive forces.
Yes, we must strive for worldwide peace and prevention of any further use of such destructive means.
I cannot fathom the destructive potential of hydrogen bombs, it is so much greater than atomic bombs
The depth of destruction brought forth while using atomic and hydrogen bombs is definitely a matter to consider on a global scale.
The detonation of atomic and hydrogen bombs have such destructive power and are both catastrophic. It is saddening
The uses of hydrogen bombs are absolutely terrifying. It reminds me of the inevitable destructive power of such weaponry.
The development and use of atomic and hydrogen bombs have certainly shaped the world we live in today.