Metals when kept uncovered, respond with air dampness, acids, and different gas present in the climate and get consumed. This interaction is called corrosion.
Food containing fats and oil when presented to the air gets oxidized and gives an awful taste and smell to food. This interaction is called rancidity.
Key Takeaways
- Corrosion is the degradation of metals due to chemical reactions, while rancidity is the spoilage of fats and oils in food.
- Corrosion can be prevented through various methods, such as coatings or galvanization, whereas rancidity can be delayed by using antioxidants or proper storage.
- Both corrosion and rancidity result in the deterioration of materials and can negatively impact performance, safety, and aesthetics.
Corrosion vs Rancidity
Corrosion is the gradual degradation or breaking down of materials, metals. It is caused by moisture or exposure to wet weather like rain, sleet, or snow. Rancidity mainly happens in food; it is the reaction of the fat in food with oxygen. Rancidity happens more rapidly.

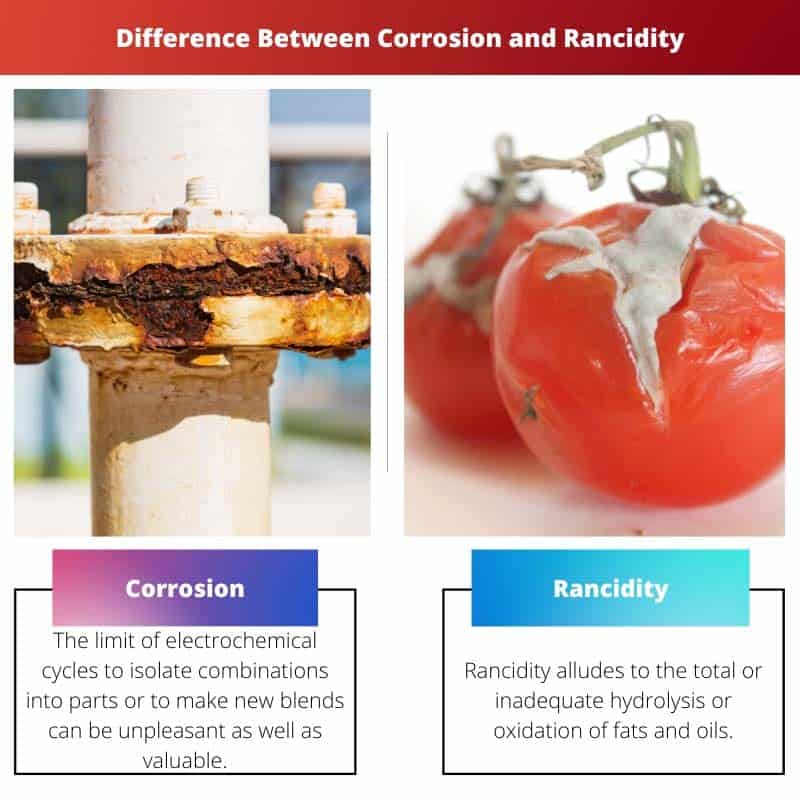
The limit of electrochemical cycles to isolate combinations into parts or to make new blends can be unpleasant as well as valuable.
Corrosion is an inside and out the ordinary eventual outcome of electrochemical reactions among materials and substances in their present situation.
Consumption is a dangerous and extremely extravagant issue. Because of it, designs and platforms can fall, oil pipelines break, engineered plants open, and washrooms flood.
Rancidity alludes to the total or inadequate hydrolysis or oxidation of fats and oils when presented to air, light, dampness, and bacterial action; this for the most part happens in food things making them unwanted for utilization.
In more broad terms, rancidity is connected all of the time with food items that contain oil and unsaturated fats. Unsaturated fats are only fats, cholesterol, and steroids.
Comparison Table
| Parameters of Comparison | Corrosion | Rancidity |
|---|---|---|
| Happenings | Happens with metals. | Happens with food. |
| Occurrence | The reaction of oxygen with metal. | The reaction of oxygen with fats in eatables. |
| Time | Slower process | Faster process |
| Example | Rusting of iron | The butter kept out in the open for too long. |
| Changes | Physical changes. | Horrible smell and impossible to eat. |
What is Corrosion?
Metal consumes when it responds with another substance like oxygen, hydrogen, and electrical flow, or even soil and microscopic organisms.
Corrosion can likewise happen when metals like steel are put under a lot of pressure making the material break.
The most widely recognized sort of iron corrosion happens when it is presented to oxygen and the presence of water, which makes a red iron oxide called rust.
Rust can likewise influence iron composites like steel.
The rusting of iron can likewise happen when iron responds with chloride in an oxygen-denied climate, while green rust, which is one more kind of corrosion, can be framed straightforwardly from metallic iron or iron hydroxide.
Corrosion of metals and nonmetals happens because of the continuous ecological association on the material surface.
The constructions and offices of various materials are impacted by this collaboration. Indeed, even the encompassing air, weighed down with dampness and oxygen, can begin this interaction, known as rusting, on steel surfaces.
The vapor of acids, for example, sulfuric corrosive and residue of burning soft drink additionally speed up corrosion.
On account of aluminum, in any case, the oxide film-shaped because of the underlying destructive assault shields the surface from additional harm.
In marine conditions, in which airborne salt gems are saved onto ships, corrosion of lowered surfaces, as well as surfaces drifting above water level, happens.
Corrosion influences the microstructure, mechanical properties, and actual appearance of the materials. Rusting and different kinds of decay diminish the limit of pipelines and hardware, bringing about loss of result as well as loss of gear, or even life.

What is Rancidity?
It is fundamental to keep food items from rancidity to hold their helpful characteristics. Unsaturated fats are only fats, cholesterol, and steroids.
These are carboxylic acids, with a long aliphatic chain. They can be either soaked, containing a solitary linkage between carbon molecules, or unsaturated, with numerous linkages between carbon particles.
Probably the most straightforward method for forestalling food items is to get them far from direct contact with light and air.
For this reason, they can be put away in water/airproof compartments. Adding cell reinforcements is additionally a powerful method for forestalling auto-oxidation activity in food sources containing fats and oils.
Cell reinforcements can be either regular or engineered. Normal cell reinforcements incorporate L-ascorbic acid, vitamin E, flavonoids, and polyphenols.
Sequestering specialists like EDTA likewise forestall or dial back oxidation and along these lines can forestall rancidity.
A genuine model is Linseed oil (transformed into smelly oil) since it tends to be utilized to solidify oil-based paints (for instance, craftsman’s ‘oil’ paints).
Likewise, the solidifying response results from the polymer arrangement of these oxygenated unsaturated fats. It is likewise accepted that pecan oil turns rank quickly.
For this specific explanation, polyunsaturated unsaturated fat oils ought to be put away in the fridge to dial back the response.
Likewise, certain individuals are touchy to pecans that have gone even somewhat smelly. The possibility that some kind of polymers framing in the stomach is especially ghastly, and one won’t eat any pecans that smell a tiny smidgen ‘off.’
Main Differences Between Corrosion and Rancidity
- Corrosion is connected with metals while rancidity is connected with food sources.
- Corrosion comprises the contact of the metals with dampness and oxygen while the rancidity implies the response of fats in food with the oxygen.
- Additionally, the rancidity is a more fast cycle than that of corrosion.
- For instance, rusting is a type of erosion wherein iron is eaten up by the activity of air and dampness, and a ruddy earthy colored covering of iron oxide is framed and for instance in rancidity when margarine is saved open for quite a while then its smell and taste gets changed.
- The changes after corrosion are mostly physical, whereas rancidity has a more severe effect like bad smell and taste.