When an element and a compound react with one another, a chemical reaction takes place. And this reaction forms a whole new product on its own.
This reaction also changes the arrangements of the atoms in every element and the compound.
Hence it either absorbs the energy or releases the energy and this is done to bring around the changes and make the reaction happen.
Based on this whole procedure and occurrence, a chemical reaction is further divided into two parts- exothermic reaction and endothermic reaction.
Key Takeaways
- An exothermic reaction is a chemical reaction that releases heat to its surroundings, while an endothermic reaction is a chemical reaction that absorbs heat from its surroundings.
- In an exothermic reaction, the energy released during the reaction is in the form of heat and light, while in an endothermic reaction, the energy absorbed is in the form of heat.
- The combustion of fuels is an example of an exothermic reaction, while photosynthesis is an example of an endothermic reaction.
Exothermic vs Endothermic Reaction
The difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat that is absorbed from the surroundings itself, while an exothermic reaction, on the other hand, releases the energy in its surrounding. An example of an exothermic reaction can be respiration, while an example of an endothermic reaction is the melting of ice.

An exothermic reaction is a form of chemical reaction that releases energy in the surrounding environment when it occurs.
The energy that is released during the exothermic reaction is more than the energy that is required to begin or even to carry out the whole chemical reaction.
The energy that is being released in the environment is basically in the form of heat or light.
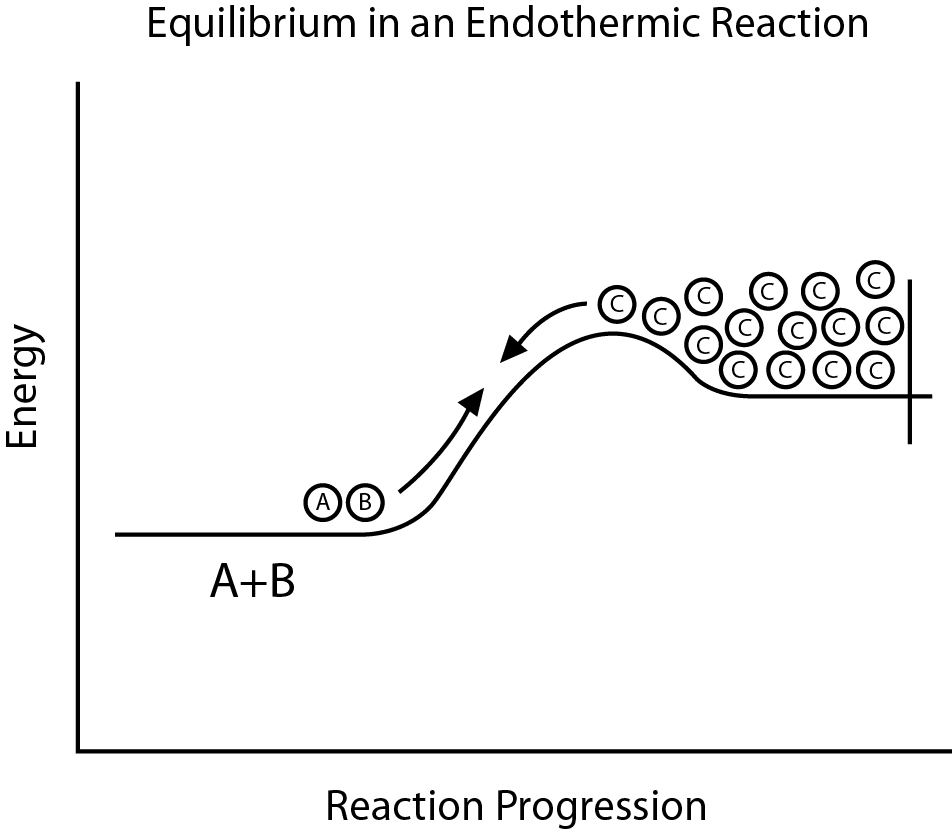
Chemical reactions are of two different types, and one of them is Endothermic reaction, where the reactants that are part of that chemical reaction take up energy from the surroundings to carry out the reaction, and that energy is in the form of heat only. The temperature of the environment gets cooled when an endothermic reaction happens. The exothermic reaction is the opposite of the endothermic reaction.
Both of them are parts of a chemical reaction.
Comparison Table
| Parameters of Comparison | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Definition | Exothermic reactions are those reactions where the energy is released into the environment. If any process requires to heat the other ones should give off the heat when they are taking place. These are known as exothermic. | Endothermic reactions are those reactions where energy is absorbed from the environment. An endothermic reaction is a part of a chemical reaction that takes place in the environment. |
| Enthalpy | In an exothermic reaction, the change in enthalpy is negative. | In an endothermic reaction, the change in enthalpy is positive. |
| Form of energy | In an exothermic reaction, energy is released in many forms say- heat, light, electricity, etc. | In an endothermic reaction, energy is released in the form of heat only. |
| Example | Respiration. | Melting of ice. |
| Energy | Energy is released in the environment in any form in an endothermic reaction. | Energy is absorbed from the environment in an endothermic reaction. |
What is Exothermic Reaction?
The exothermic reaction releases all the heat into its surrounding environment and also replaces the weak bonds with the stronger ones at the same time.
There is another form of reaction named exergonic reaction, with which exothermic reaction is confused too. However, a very strong exothermic reaction can be called exergonic, but not all the time.
Basically, if any process requires heat, the other ones should give off the heat when they are taking place. These are known as exothermic.
For instance- steam which is nothing but the gaseous form of water condenses, and heat is released in the surrounding environment. Similarly, when water in its liquid form freezes, heat is given off in the environment.
The liquid water had to have energy out into it to become steam, but that energy is not at all lost in the environment.

What is Endothermic Reaction?
An increase in enthalpy is nothing but an endothermic reaction of a system. An endothermic reaction is part of a chemical reaction that takes place in the environment.
Talking of any closed system, what the reaction makes happen in the environment is it absorbs the thermal energy from its surroundings which is actually nothing but heat transferred into the same system of the reaction during the process.
When an endothermic reaction occurs, the environment around it gets cooled, and this is because of the reason that heat energy is absorbed into the reaction.
An endothermic reaction is not a major or extraordinary reaction. In fact, it can be a simple chemical process too, for example- dissolving ammonium nitrate in water, or even any physical process that requires heat to be released, like the melting of ice. Both of them are parts of a chemical reaction. Therefore, in each of these terms, the prefix of the word depends on heat, whether it comes out or it gets released.
Main Differences Between Exothermic and Endothermic Reaction
- The main difference between Exothermic and Endothermic reaction is that Exothermic reactions are those reactions which release energy in the environment when it occurs, while endothermic reactions, on the other hand, are those which absorbs energy from their surrounding environment in order to carry out.
- The change in enthalpy is negative in an exothermic reaction, while in an endothermic reaction, the change in enthalpy is positive.
- Energy can be released in any form, say heat, light, electricity, etc., when an exothermic reaction takes place, but in an endothermic reaction, energy is released in only one single form, and that is heated.
- An example of an exothermic reaction would be Respiration, while an example of an endothermic reaction would be the Melting of ice.
- In an endothermic reaction, energy is released into the environment, while in an endothermic reaction, energy is absorbed from the environment.
The detailed explanation of exothermic and endothermic reactions in the article is beneficial for students and anyone interested in chemistry.
I found the content to be well-structured and informative, providing a clear understanding of how these reactions occur.
The article explains the concept of exothermic and endothermic reactions very clearly and concisely. It’s a great resource for anyone wanting to understand these concepts in-depth.
It certainly provides a solid foundation for understanding these fundamental chemical reactions.
This article effectively explains the concepts of exothermic and endothermic reactions, providing clear examples and a thorough comparison of their properties.
The article is a valuable resource for gaining a deeper understanding of exothermic and endothermic reactions in chemistry.
It’s a well-written piece that delves into the complexities of exothermic and endothermic reactions, making it an engaging read.
The article provides a comprehensive overview of exothermic and endothermic reactions, furthering the reader’s understanding of the complex processes involved in chemical reactions.
I found the examples of exothermic and endothermic reactions particularly helpful in clarifying the differences between the two.
The detailed examples and comparison table make the differences between exothermic and endothermic reactions very clear, allowing for a better understanding of their properties.
The practical examples provided in the article support a comprehensive understanding of exothermic and endothermic reactions.
The logical flow of the article helps to succinctly outline the key concepts underpinning exothermic and endothermic reactions.
I appreciate the thorough comparison between exothermic and endothermic reactions, making it easier to comprehend the differences and applications of each type of reaction.
The article effectively captures the essence of exothermic and endothermic reactions, providing valuable insights into their significance.
The article brilliantly elaborates on exothermic and endothermic reactions, highlighting their significance in understanding chemical processes.
A very interesting article explaining the differences between exothermic and endothermic reactions, as well as how chemical reactions take place. The examples provided are very illustrative.
I enjoyed reading this article, it’s a great refresher on basic chemistry principles.
I agree, it’s a very comprehensive and enlightening explanation.
This article offers an insightful exploration of exothermic and endothermic reactions, contributing to a deeper understanding of these fundamental chemical phenomena.
The article’s comprehensive coverage of exothermic and endothermic reactions enables readers to grasp the complexities of chemical reactions more effectively.
The article offers a well-organized and insightful explanation of exothermic and endothermic reactions, delivering a valuable learning experience.
The in-depth analysis of thermal energy transfer in exothermic and endothermic reactions greatly contributes to understanding these fundamental chemical processes.