Substances around us undergo changes that may not always be noticeable to us. It is difficult to classify them as physical and chemical changes.
Regardless, both have major differences between them that might make it easy to understand the concepts. Furthermore, they might also help decipher each of them.
Key Takeaways
- A physical change involves a change in the physical properties of a substance without altering its chemical composition.
- Chemical change involves a change in the chemical composition of a substance, resulting in the formation of a new substance.
- Physical changes are reversible, while chemical changes are irreversible.
Physical vs Chemical Change
Physical change is a situation where the physical composition of an object or individual changes. But its chemical properties stay the same, and it is temporary. Chemical change is the process where the chemical properties of an object or person change entirely, and it is always permanent.

When an object undergoes a physical change, its appearance shows noticeable differences. The object however remains the same even though its physical properties are completely transposed.
New energy is not produced during the process. Some examples of physical change include freezing water, shaping clay, and even chopping vegetables.
When an object undergoes a chemical change, an entirely new substance is created that now has a different chemical composition, unlike the former, this kind of transposition is permanent.
New energy tends to be produced during the process. Some examples of chemical change include iron rusting, burning coal, and cooking vegetables.
Comparison Table
| Parameters of Comparison | Physical Change | Chemical Change |
|---|---|---|
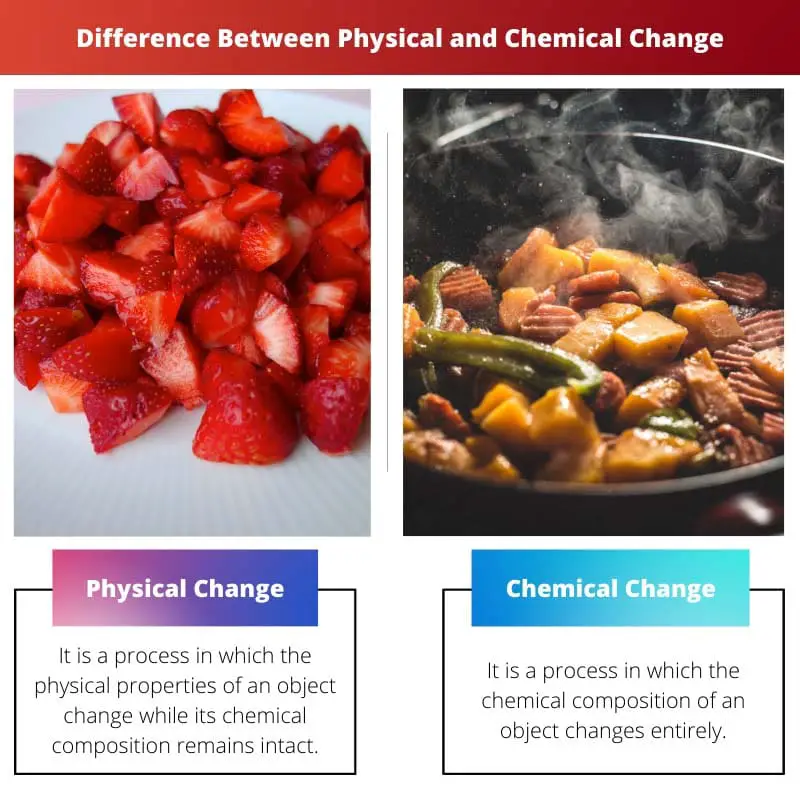
| Definition | It is a process in which the physical properties of an object change while its chemical composition remains intact. | It is a process in which the chemical composition of an object changes entirely. |
| Effects | The appearance of the object changes. | The appearance of the object changes along with its chemical bonds. |
| Nature | It is a temporary change. | It is a permanent change. |
| Energy Production | New energy is not produced during the process. | New energy is produced during the process. |
| Energy Absorption | Very little to no energy is absorbed by the object. | A substantial amount of energy is absorbed, which then evolves. |
| Result | A new substance is not formed. | A new substance is formed. |
| Reversibility | It is easily reversible. | It is not reversible. |
| Examples | Some examples include freezing water, shaping clay, and chopping vegetables. | Some examples include rusting of iron, burning of coal, and cooking of vegetables. |
What is Physical Change?
Physical change is a process in which the physical properties of a substance change while its chemical composition is retained. Result, the appearance and form of the substance change entirely.
This includes its shape, size and, in some cases, even how it smells. When a substance undergoes the process, the result is reversible.
New substances with a different chemical composition are not formed after a physical change occurs. In fact, very little to no energy is absorbed during the process.
Neither is any form of energy created. It is only that the state of matter of the substance changes from one form to the other.
A great example of a physical change is grinding a piece of wood into sawdust. Once the process has taken place, the result involves a change in appearance.
Even though the substance’s state changes, the wood’s chemical composition remains the same. Moreover, there is no exchange, conversion or production of any kind of energy.
Other examples of physical changes include the melting of ice and boiling of water. Breaking a glass, tearing a paper bag, crumpling paper, chopping wood.
Mixing sand with water, mixing red stones with green-coloured stones, and even cutting vegetables.
What is Chemical Change?
A chemical change is a process in which the chemical bonds in an object are broken, to form an entirely new substance. It is called a chemical reaction.
Through the process, the appearance as well the chemical composition of the object change. The result is not reversible.
A significant amount of energy is absorbed and produced during a chemical change. After undergoing a chemical change, an entirely new substance is formed.
The process may be organic, inorganic, or biochemical. An organic process is that which involves carbon. Inorganic changes do not involve or associate with carbon.
Meanwhile, biochemical changes involve the chemistry of growth and survival of a living organism. Some evidence of chemical changes includes a change of odour, emitted light or heat.
Creation of gases, decomposition of matter, formation of bubbles, change in temperature and even change in colour. When any of these occur during, say, an experiment.
It means that a chemical change has taken place. Examples of chemical changes include food digestion, rusting of iron, cooking food, baking cookies, curdling of milk, wood burning in a furnace,
Ripening of fruits, fermentation, photosynthesis and even fire explosions. These bring a permanent change in the chemical composition of objects and create a new substance with a different chemical.
Main Differences Between Physical and Chemical Change
- Physical change is a process in which the physical properties of an object change while its chemical composition remains intact, whereas chemical change is a process in which the chemical composition of an object changes entirely.
- Physical change brings a change in appearance, whereas a chemical change brings a change in appearance and chemical bonds.
- Physical change is temporary, whereas chemical change is permanent.
- A physical change does not create new energy, whereas a chemical change creates it.
- A physical change involves little to no absorption of energy, whereas a chemical change involves a significant amount of energy.
- A physical change does not create a new substance, whereas a chemical change does.
- Physical change is easily reversible, whereas chemical change is irreversible.
- Examples of Physical change include freezing of water, shaping of clay, and chopping vegetables, whereas chemical change includes rusting of iron, burning of coal, and cooking of vegetables.
- https://onlinelibrary.wiley.com/doi/abs/10.1002/jsfa.2740230406
- https://www.sciencedirect.com/science/article/pii/S0260877404003322
