As there is a lot of confusion between the terms fractional distillation and simple distillation, so many people consider it the same term with a different name.
This is the process in which chemical compounds are part of the boiling to a temperature from which one or more fractions of the mixture will evaporate. It is the method that uses distillation to fractionate.
The method can be used to part the liquid with at least fifty degrees difference in boiling temperature. Water distillation is one example of simple distillation.
Key Takeaways
- Fractional distillation separates components of a mixture based on differing boiling points using a fractionating column.
- Simple distillation involves heating a mixture and collecting the vapor, which is ideal for separating substances with significantly different boiling points.
- Fractional distillation is more efficient for mixtures with close boiling points, while simple distillation suits mixtures with large boiling point differences.
Fractional Distillation vs Simple Distillation
In simple distillation, two liquids with significantly different boiling points are separated. When a liquid with a lower boiling point vaporizes first, the other liquid is left behind for condensation. Using a tube, fractional distillation separates liquids with the same boiling point.

Comparison Table
| Parameter of Comparison | Fractional distillation | Simple distillation |
|---|---|---|
| Meaning | Fractional distillation is the process of separating the mixture into its parts. | Simple distillation is the process of separating two liquids with different boiling points. |
| Boiling point | Fractional distillation can separate the mixture, which has boiling points close to each other. | Simple distillation can separate the mixture fifty degrees difference in their boiling nature. |
| Example | Fractional distillation is used for crude refining. | Simple distillation is used to purify seawater. |
| Used for | Regarding the use case, Fractional distillation uses a complex apparatus with a fractionating column. | Talking about the used case, simple distillation uses a complex apparatus with a fractionating column. |
| Repetition | In fractional distillation, this process has to be done many times to get a pure component. | Simple distillation is when we get pure components for the first time. |
What is Fractional Distillation?
Federal distillation separates chemical compounds from each other when the boiling temperature difference is less than forty degrees.
This process ensures that the condensation teaches to its final condenser as soon as possible. One example of fractional distillation is crude oil split into various components.
Fractional distillation uses a fractionating column because the liquid mixture has a similar boiling point. The fractionating column acts as a hurdle to the rising gas.
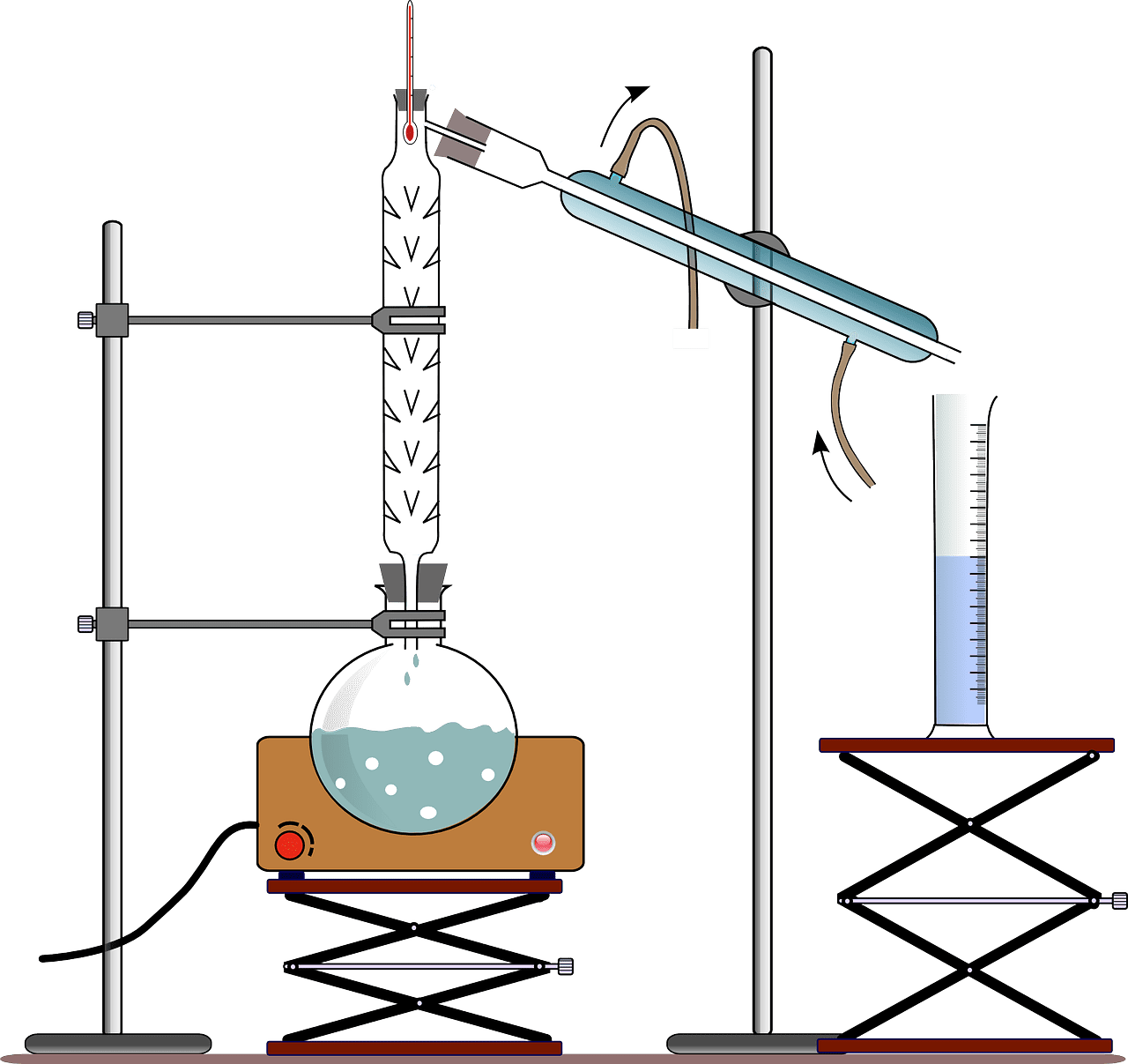
What is Simple distillation?
Simple distillation is the method of splitting up two-component which have different boiling points. It can split up liquids with at least fifty degrees different boiling points.
Refine compounds will be warm-up and turned into condensation at a relatively low-temperature range of 2 or 3 degrees Celsius; by looking at the distillation flask’s temperature, it is possible to affect a good split up in the compound.
This process of simple distillation will continue until the original mixture is separated. Simple distillation is best for liquid with a different boiling point.
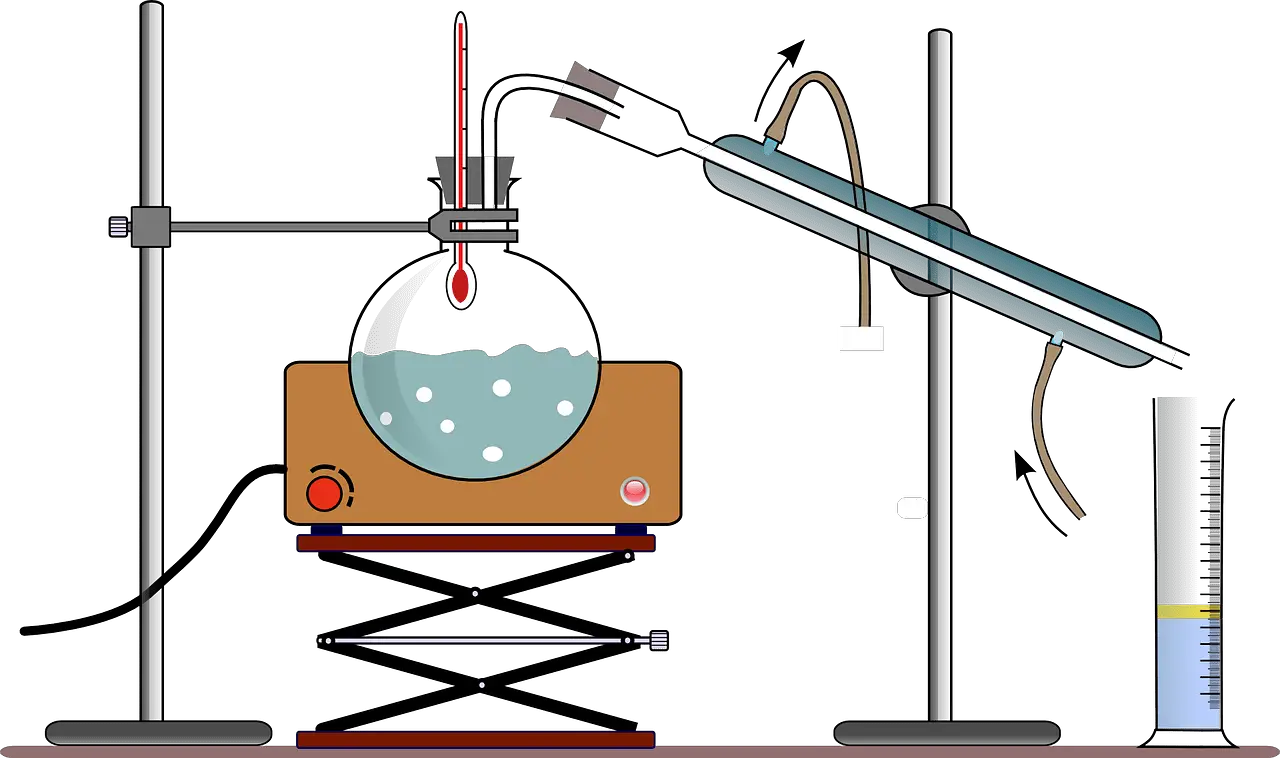
Main Differences Between Fractional Distillation and Simple Distillation
- Both simple and fractional distillation processes are all about splitting up different properties of liquids.
- If two or more liquids have a close boiling point to each other, then we can separate them by fractional distillation; on the other hand, simple distillation is the process of separating liquid, which has a fifty-degree difference in boiling point.
- https://aocs.onlinelibrary.wiley.com/doi/abs/10.1007/BF02667433
- https://pubs.acs.org/doi/pdf/10.1021/ac60061a012

The article provides a clear distinction between fractional and simple distillation methods.
The clarity in the comparison is commendable.
Certainly, the distinction is elucidated effectively.
The distinction between the two distillation methods is well-defined in the article.
Certainly, the comparison is clearly articulated.
An interesting article, yet it could have delved deeper into the scientific nuances of the methods.
The scientific intricacies could have been more thoroughly explored.
I agree, more scientific depth would have added value to the comparison.
The article is written in a rather simplistic manner.
Yes, a more in-depth approach would have been appreciated.
The comparison could have been presented in a more engaging manner.
Agreed, a more engaging presentation would have enhanced the article.
The article is lacking in terms of engaging content.
This article summarises the differences and uses of fractional and simple distillation precisely, an interesting read.
Indeed! It provides a clear understanding of the topic.
An interesting take on the comparison between fractional and simple distillation methods.
Yes, the practical examples are quite helpful in clarifying the concepts.
The use of practical examples makes it easier to understand.
An informative article that gives a detailed comparison of fractional and simple distillation methods.
The detailed comparison of the methods is commendable.
The examples provided add depth to the comparison.
The article lacks a scientific approach to the explanation.
Indeed, the scientific principles could have been explained more clearly.
The article does not provide a comprehensive explanation of the differences.
I agree, it lacks depth and thoroughness.