Chemistry’s periodic table is something students are being asked to memorize tediously without even knowing the actual motive behind learning it. For some people, it’s just a topic included in their syllabus.
But in a real sense, this little periodic table is way more important than that; it is a roadmap that unlocks a million opportunities for scientists and researchers worldwide. Dmitri Mendeleyev is the inventor of the periodic table.
Before him, many tried hard to arrange chemical elements in different ways. But Dmitri’s outcome was accepted worldwide.
In scientific abbreviations, the rows are referred to as Periods and the columns as Groups, respectively.
Key Takeaways
- Periods are the horizontal rows in the periodic table, indicating the number of electron shells in an element’s atoms, with elements in the same period having similar atomic structures.
- Groups are the vertical columns in the periodic table, consisting of elements with the same number of electrons in their outer shell, which leads to similar chemical properties.
- Both periods and groups organize elements in the periodic table, with periods representing horizontal rows based on electron shells and groups representing vertical columns based on outer shell electrons and chemical properties.
Period vs Group
A period is a horizontal row that goes from the left side to the right side of the periodic table, and electronegativity increases across it. A group is a vertical column that goes from the top of the periodic table to the bottom, and electronegativity increases from bottom to top.
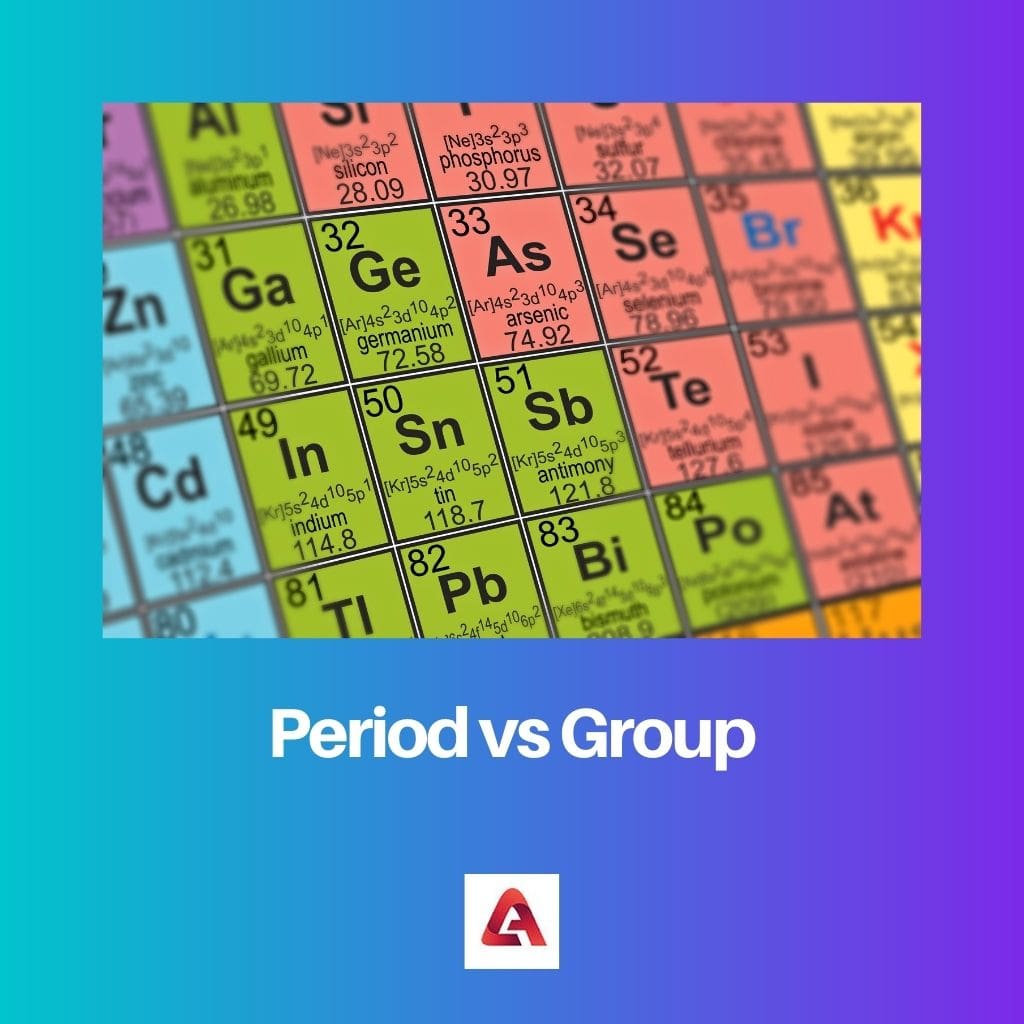
During the time of arranging, Mendeleyev left a few rows empty with the impression that some other elements would enter in the near future. And surprisingly, one of the elements that fitted in that gap was Gallium.
Comparison Table
| Parameter of Comparison | Period | Group |
|---|---|---|
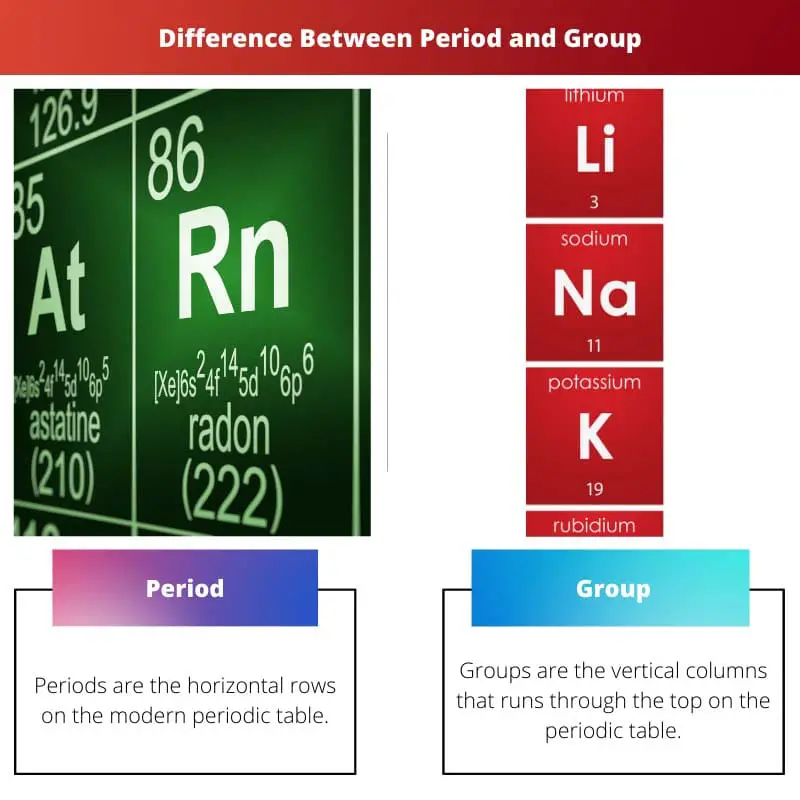
| Direction | Periods are the horizontal rows on the modern periodic table | Groups are the vertical columns that run through the top of the periodic table |
| Properties | The elements in a period do not have similar properties. | The elements in each group have some similar properties but not identical properties. |
| Similarity | Elements in the same period have an equal number of electron composition | Elements in each group have an equal number of valence electrons |
| Sum | There are 7 periods on the periodic table | The group contains 18 elements arranged vertically in the modern periodic table. |
| Electronegativity | It increases from left to right. | It increases from bottom to top in a group. |
What is Period?
A period is a horizontal row from extreme left to far right on the periodic table. As of now, there are 7 periods on the periodic table.
A new period begins when a fresh fundamental energy level adds up with the electrons. Every element in a period will likely have an equal number of atomic orbitals.
For example – every element in the 1st period has only 1 orbital for its electrons, 2nd period includes 2 orbitals for the electrons. Similarly, orbitals keep adding up as you move down in the row.
The element’s size decreases as you move across a period as the number of electron shells remains constant, but the number of protons rises in the nucleus. This is why the atom gets heavier, but the size keeps decreasing.
Looking at the periodic table, you would see different elements fitted in each row. The 1st period has only 2 elements (1 & 18), the 2nd and 3rd periods have 8 elements each, the 4th and 5th periods have 18 elements, and the 6th and 7th periods have 32 elements each, respectively.
What is Group?
Counting from top to bottom, there are 18 groups in the periodic table. All the groups are allotted distinct names.
The groups are mixed categories of metals, non-metals, and semi-metals, grouped into families according to their similar properties. E.g., Group 1 belongs to the Lithium family, classified as Alkene metals.
Similarly, each group in the lane has its family name. Elements in the related group have similar traits because they have the same electron counts in their outermost shells.
The size of the element increases as you move any group downwards. This is because large numbers of protons and neutrons exist in the nucleus.
On top of this, an extra electron shell makes the atom heavier. For groups, there are two different ways to illustrate the elements.
Understanding both numbering systems is essential because the periodic table appears in both formats. In the United States, they used the letters A&B to indicate each element in the group, but unfortunately, it was observed as a disorganized numbering system.
To eliminate all possible confusion, the International Union of Pure and Applied Chemistry (IUPAC) Came up with the idea of numbering the elements as (1,2, 3… 18). However, both numbering systems are acceptable. But IUPAC’s numbering looks well-organised and straightforward.
Main Differences Between Period and Group
- Location: Groups are the upright column, whereas Periods are the straight rows in the periodic table.
- Number: There are a total 18 number of groups and 7 periods in the periodic table, among which the groups are categorized under different families and metal types.
- Chemical properties: All the elements in the group have analogous chemical or physical properties, whereas periods share the same electron hierarchy.
- Energy Level: As we go down the group from top to bottom, the energy level of electrons increases. On the other hand, in each period, the electron’s energy level remains the same.
- Electronegativity: This declines from top to bottom in a group and rises from left to right in a period. This is an essential consideration while studying the periodic table.

Great article! This gives a great insight into the importance of the periodic table. I really enjoyed it!
I’m very glad you found it helpful. I did too!
Absolutely, every science student should read this.
Very informative post. We should push for better education and understanding of scientific topics.
This is exactly what we need.
This is just another example of outdated educational standards. We should be teaching more relevant topics to students.
I believe that it’s of vital importance for students to understand the periodic table.
That’s a controversial take, Joel.
I think people underestimate the value of the periodic table. We should be doing more to enhance our teaching of it.
Absolutely, this is an important point.
I think it’s really interesting that such a seemingly simple table can hold so much valuable information.
Yes, it’s quite fascinating.
This is enlightening. The periodic table is underestimated.
This was a much needed read to start my day!
Some valid points presented here, but I’m still not convinced of the importance of the periodic table.
I agree with you, Zach.
The periodic table is a cornerstone of chemistry and should be treated as such.
I couldn’t agree more.
I disagree, I believe that teaching the periodic table in the current way is outdated and should be revised.
I can see why some might find it outdated, but it really is vital to scientific development.
I can see what you’re saying but I don’t fully agree.
While I understand the importance of the periodic table, it’s hard to argue that the current educational system is adequate.
You raise an important point, Erin.