A human body consists of several enzymes, cells, tissues, organs, processes etc. Each of these elements performs its independent function and helps in the working of the body.
The human body stays fit and healthy if all these elements work smoothly. The vital proteins and other minerals that are required for a human body are provided by these cells, and one of those molecules present in the human body is DNA.
There are several parts and elements present in DNA. However, the nitrogen bases present in both DNA and RNA are 1. Purines, and 2. Pyrimidines.
Key Takeaways
- Purines are nitrogen-containing compounds consisting of two rings, while pyrimidines are nitrogen-containing compounds consisting of one ring.
- The DNA molecule contains both purines and pyrimidines.
- Adenine and guanine are purines, while thymine, cytosine, and uracil are pyrimidines.
Purines vs Pyrimidines
Pyrimidines (cytosine, thymine, and uracil) are single-ring nitrogenous bases found in DNA and RNA, while purines (adenine and guanine) are double-ring nitrogenous bases found in DNA, RNA, and ATP. Purines are larger and have a more complex structure than pyrimidines.
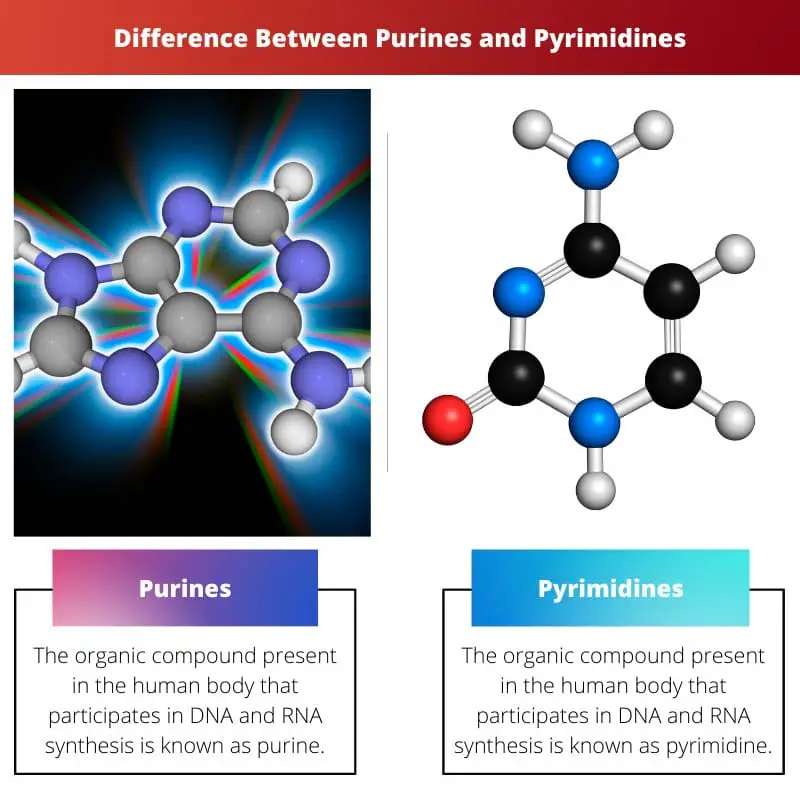
The organic compound present in the human body that participates in DNA and RNA synthesis is known as purine. It is a heterocyclic aromatic organic compound that consists of nucleobases like adenine and guanine.
The aromatic ring is made up of four nitrogen atoms and two hydrogen-carbon rings.
The organic compound present in the human body that participates in DNA and RNA synthesis is known as pyrimidine. They form the nitrogen base and are also addressed as building blocks.
It consists of nucleobases like thymine, uracil and cytosine. It is made up of two atoms of nitrogen and one hydrogen-carbon ring.
Comparison Table
| Parameters of Comparison | Purines | Pyrimidines |
|---|---|---|
| Consists of | Four nitrogen atoms, two hydrogen-carbon rings, adenine and guanine. | Two nitrogen atoms, one hydrogen-carbon ring, thymine, cytosine, and uracil. |
| Melting points | 214 Degrees Celsius | 20-22 Degrees Celsius |
| Catabolism | Uric acid is produced. | Ammonia, Carbon Dioxide and beta-amino acids are produced. |
| Complexity | The synthesis process is complex. | The synthesis process is simpler. |
| Major Pathway | Salvage | De Novo |
What is Purines?
Purine is a form of organic compound that is present in the human body. It plays an important role during the synthesis of DNA (Deoxyribonucleic acid) and RNA (Ribonucleic acid).
The IUPAC name of purine is “9H-Purine”. Other than the human body, purines are also found in other organisms.
Purines are weak acids as well as weak bases. However, they are weaker bases.
The term “purine” was first tossed by Emil Fischer. In 1884, the term was used for the first time, and the synthesis experiment was done in 1898 for the first time.
During the experiment, uric acid was used as a starting material.
They play an important role in the synthesis of DNA and RNA, but they also play an important role in other biomolecules. These biomolecules where purines function includes ATP, coenzyme A etc.
Other than that, they can also be produced by means of organic synthesis, irrespective of the fact that they can not be found naturally.
Purines play an important role in the production of proteins and starch. They provide the necessary elegy for the cells in the human body.
They are also important for the signalling of the cells and the regulation of enzymes. The purines are synthesized biologically, and if they accumulate, they are very defective to various other cell processes inside human bodies.

What are Pyrimidines?
Pyrimidine is a form of organic compound that is present in the human body. They and purines play an important role in DNA and RNA synthesis.
The systematic IUPAC name of pyrimidine is “1,3-Diazabenzene”. There are three nucleobases present in pyrimidines, namely, thymine, uracil and cytosine.
Initially, the study of pyrimidine began in the year 1884. The derivatives were synthesised with the help of condensation of ethyl acetoacetate and amidines.
One year later, in 1885, the term “pyrimidine” was tossed for the first time by Pinner. Many other scientists did a deep study of pyrimidine later on.
The distinguishing feature of cytosine, a pyrimidine base, is by having an amine group at the fourth position and a keto group at the second position. It matches with guanine in the DNA and RNA.
Three hydrogen bonds pair cytosine and guanine. This doesn’t is further transformed into CTP or cytidine triphosphate when three phosphoric acid groups are used to phosphorylate them.
Another pyrimidine base is thymine. In the heterocyclic aromatic ring of thymine, two keto groups occupy the second and fourth positions, and a methyl group occupies the fifth position.
In DNA and RNA, thymine matches with adenine. They are paired with two hydrogen bonds.
Alike thymine, uracil too matches with adenine.

Main Differences Between Purines and Pyrimidines
- Purines consist of nucleobases like adenine and guanine; on the other hand, pyrimidines consist of nucleobases like thymine, uracil and cytosine.
- In the heterocyclic aromatic ring of purines, there are two hydrogen-carbon rings. On the other hand, in the heterocyclic aromatic ring of pyrimidines, there is one hydrogen-carbon ring.
- In the heterocyclic aromatic ring of purines, there are four nitrogen atoms. On the other hand, there are two nitrogen atoms in the heterocyclic aromatic ring of pyrimidines.
- During catabolism in purines, uric acid is produced. On the other hand, during catabolism in pyrimidines, ammonia, beta-amino acids and carbon dioxide is produced.
- The major pathway in purines is salvage. On the other hand, the major pathway in pyrimidines is De Novo.
- The synthesis process is complex in purines. On the other hand, the synthesis process is simpler in pyrimidines.
- https://pharmrev.aspetjournals.org/content/50/3/413.short
- https://link.springer.com/article/10.1007/BF00928361

It is fascinating to see the structural differences between purines and pyrimidines and how they affect the process in the human body. Great article!
Thank you for sharing this in-depth analysis. The information about the production and functions of purines and pyrimidines has been beneficial in gaining a better understanding of their biological roles.
The detailed information about the synthesis and roles of purines is very informative. It’s interesting to learn about their functions beyond DNA and RNA. Thank you!
I appreciate the comprehensive overview of pyrimidines and their key role in DNA and RNA synthesis. The background on their study and discovery adds depth to the article.
Thanks for the explanation. It seems that purines and pyrimidines are important organic compounds that can be found naturally in the human body and play a significant role in DNA and RNA synthesis.
The main differences between purines and pyrimidines are clearly outlined, providing an insightful comparison. Well-written piece.
The comparison table of purines and pyrimidines provides a clear understanding of their characteristics and functions. Keep up the good work!
The examination of purines and pyrimidines from a scientific perspective is enlightening. The article effectively presents its significance in biological processes.