Many techniques are available to break down the holding up of intermolecular and intramolecular forces in a laboratory of industries and medical-related places. These forces have substances together.
Key Takeaways
- Dissolution is the process of a solid dissolving in a liquid to form a solution, while Disintegration is the process of a solid breaking down into smaller pieces.
- Dissolution involves a chemical change, while Disintegration involves a physical change.
- Dissolution is affected by temperature and solubility, while Disintegration is affected by factors such as particle size and moisture content.
Dissolution vs. Disintegration
The dissolution process can happen only after the breakdown of the substances with low solubility in them. On the other hand, disintegration can occur even without breaking down the substance with low solubility by a person in a laboratory.

Dissolution is a technique where different forms of a substance, such as gas, liquid, and solid, are made to dissolve to break down a specific substance in industries or pharmaceutical areas.
The term Disintegration is a technique where a substance is broken down into small chunks and bits of particles and molecules in a chemical industry or a pharmaceutical area.
Comparison Table
| Parameters of Comparison | Dissolution | Disintegration |
|---|---|---|
| Meaning | The dissolution process is where a substance is made to solve by breaking down into tiny particles. | The process of disintegration is where a solid substance is broken down into tiny molecules to make it soluble or increase its solubility. |
| States | The dissolution process can be tested on a gaseous, liquid, and solid substance. | The process of disintegration can be tested on solid substances only. |
| When it can be performed | The dissolution process can be performed only after a breakdown of particles of low solubility. | The process of disintegration can be performed irrespective of a substance, whether it has a high or low solubility level. |
| Function | The process of dissolution helps in dissolving substances into solutes. | The process of disintegration helps in the breakdown of a substance into tiny molecules or particles. |
| Form | The process of dissolution is a form of disintegration. | The process of disintegration is a whole big concept. |
What is Dissolution?
Dissolution is a technique where different forms of a substance, such as gas, liquid, and solid, are made to dissolve to break down a specific substance in industries or pharmaceutical areas.
It should be noted that normal water can dissolve any substance, meaning water is a universal solvent. It also must be pointed out that a gaseous solvent can be dissolved only through another gaseous solute by a person.
In the case of a solid substance, that substance can be dissolved only by shaking, but not all substances would dissolve just by shaking, and some may need to break down.
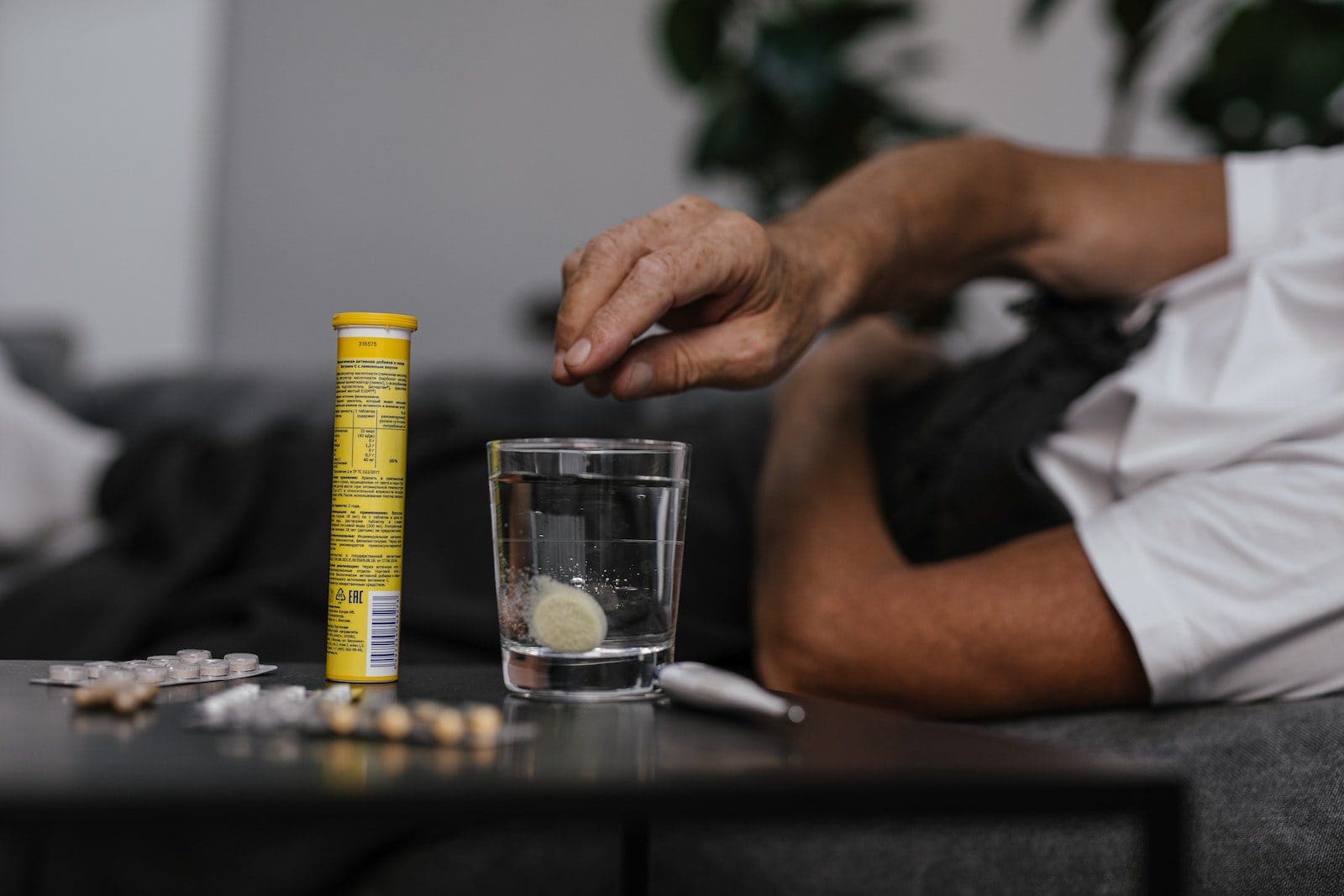
What is Disintegration?
The term Disintegration is a technique where a substance is broken down into small chunks and bits of particles and molecules in a chemical industry or a pharmaceutical area.
In chemical reactions, the compounds are made to disintegrate. Often, the dissolution process can clash with the process of disintegration. This is when water solvent gets directly soluble in small pieces of a solid substance.
For any tablet or medicine to be taken by a human, it must be dissolved or soluble. This is done through the process of disintegration.

Main Differences Between Dissolution and Disintegrated
- The process of dissolution helps in dissolving substances into solutes. On the other hand, the disintegration process helps break down a sense into tiny molecules or particles.
- The process of dissolution is a form of disintegration; on the other hand, the process of disintegration is a whole big concept.


14 thoughts on “Dissolution vs Disintegration: Difference and Comparison”
The real-life examples and comparisons presented in this article truly enhance the understanding of dissolution and disintegration processes.
The comparison table is particularly helpful to understand the clear differences between dissolution and disintegration.
Absolutely, it provides a clear and concise overview of the main distinctions between the two processes.
The process of disintegration is particularly fascinating, as the breakdown of substances into small molecules is an essential step in various chemical reactions.
The distinction between the two processes is vital, especially in the pharmaceutical and chemical industries.
I appreciate the practical examples provided to illustrate the concepts of dissolution and disintegration.
It’s interesting to learn about the specific factors that affect dissolution and disintegration processes, such as temperature, solubility, and particle size.
I appreciate the detailed explanation of dissolution and disintegration and their relevance in various industries. It’s crucial knowledge for professionals working with these processes.
I’m a bit skeptical about the practicality of these techniques in a laboratory setting. It seems like it could be complicated to put in practice, especially for substances with low solubility.
I understand your concern, but with the right instruments and understanding of the processes, it is definitely attainable and used daily in laboratory settings.
Yes, these techniques definitely require a high level of expertise, but they are essential in many industries and have been successfully implemented for many years.
This article provides a comprehensive overview of dissolution and disintegration, making it very beneficial for individuals interested in the field of chemistry and pharmaceuticals.
Very informative. It is important that the general public understands these processes that occur in many manufacturing industries and not just in the pharmaceutical area.
Absolutely, this distinction is particularly important within the pharmaceutical industry, where it is necessary to have a deep understanding of dissolution and disintegration processes.