Chemistry is a very interesting subject among every other. It includes bonds, resonance, vivid compounds, chemical reactions, etc.
In chemistry, compounds give rise to ions, cations, anions, electrons, etc. The main attraction of the subject is the periodic table that occupies all the elements found on earth, naturally or artificially created.
Key Takeaways
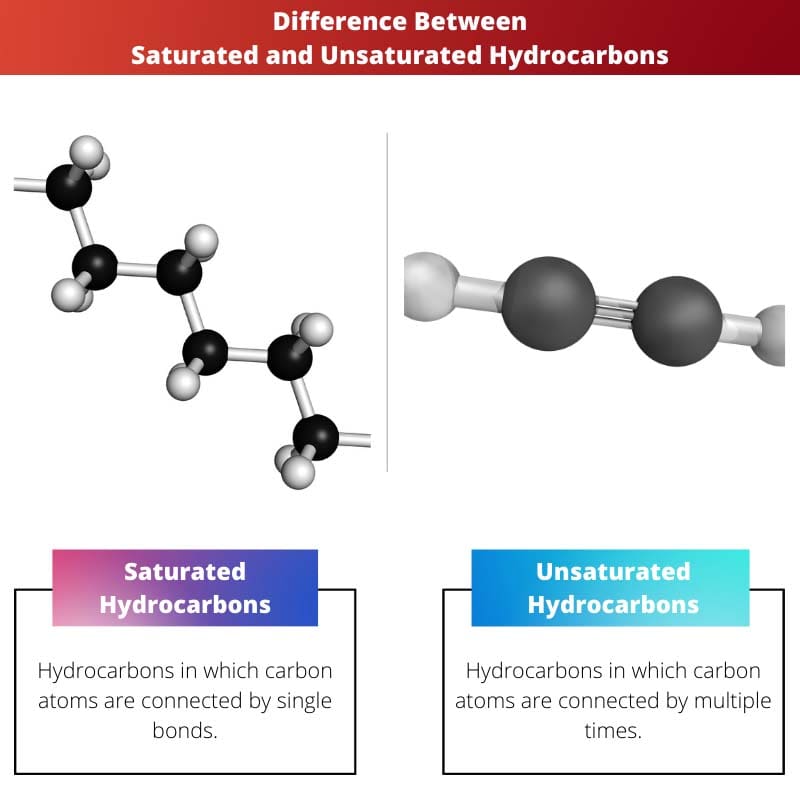
- Saturated hydrocarbons contain single bonds, while unsaturated hydrocarbons contain double or triple bonds.
- Saturated hydrocarbons are solids or liquids at room temperature, while unsaturated hydrocarbons are gases or liquids.
- Saturated hydrocarbons have a higher melting and boiling point than unsaturated hydrocarbons.
Saturated Hydrocarbon vs Unsaturated Hydrocarbon
The difference between Saturated Hydrocarbon and Unsaturated Hydrocarbon is that the compound of saturated hydrocarbons contains or has a single bond between the two carbon, i.e., the two different carbon atoms are connected via a single bond between them.

Saturated Hydrocarbon is stated as hydrocarbons made up of only carbon and hydrogen, where the two carbon atoms are attached via a single bond.
The remaining bonds of the carbon atoms are fulfilled by the hydrogen atoms so that it doesn’t form any multiple bonds. Alkanes are an example of saturated hydrocarbon.
The compounds of alkanes are – methane, ethane, butane, pentane, hexane, etc.
Unsaturated Hydrocarbon is stated as hydrocarbons are made up of only carbon and hydrogen, and the two hydrogen bonds are connected by multiple bonds.
Unsaturated hydrocarbons have two types of hydrocarbon compounds, and they are said to be alkenes and alkynes.
Examples of alkenes compounds are – butene, hexene, pentene, ethene, etc., and examples of alkynes compounds are – acetylene, oct-1-yne, 1-hexyn, etc.
Comparison Table
| Parameters of Comparison | Saturated Hydrocarbon | Unsaturated Hydrocarbon |
|---|---|---|
| Definition | Hydrocarbons in which carbon atoms are connected by single bonds | Hydrocarbons in which carbon atoms are connected by multiple times |
| Types of Hydrocarbons | Two types – Alkanes and Cycloalkanes | Three types – Alkenes, Alkynes, Aromatic |
| Hybridization | sp3 | sp2 or sp1 |
| Hydrogen Atoms | More number of atoms | Less number of atoms |
| Chemical Reactivity | Low | High |
| Flame Test | Gives a blue flame | Gives a sooty flame (or soil-colored) |
| Example | Alkanes, Cycloalkane, Butane, Pentane | Alkynes, Alkenes, Aromatic, Acetylene |
What is Saturated Hydrocarbon?
Saturated hydrocarbons are defined as the hydrocarbon formed by two carbon atoms with a single bond between them. The rest of the bonds are connected or formed by the hydrogen atoms to fulfill the carbon valency.
The general formula used to generate the saturated hydrocarbon is – CnH2n+2.
Saturated hydrocarbons are of two major types – alkanes and cycloalkanes. Some of the alkane compounds are – methane, ethane, propane, butane, pentane, etc.
Cycloalkanes are compounds with ring structures along with hybridized atoms. The properties of cycloalkanes are almost similar to that of alkanes except for the boiling and melting point, which are comparatively higher.
The following are the properties or the uses of the saturated hydrocarbons –
- Saturated hydrocarbons contain a greater number of hydrogen atoms in their compounds.
- Methane – the first alkane compound is very useful and can be used as rocket fuel or in automobiles, heaters, etc.
- Alkanes and cycloalkanes are two significantly different types of saturated hydrocarbons.
- Cycloalkanes can be used for the production of nylon, petroleum gas, rubbers, etc.
- Ethane, the second alkane compound, is the refrigerator coolant. Also, it plays a major role in the production of ethylene.
What is Unsaturated Hydrocarbon?
Unsaturated hydrocarbons are defined as the hydrocarbon formed by two carbons with multiple bonds between them. The multiple bonds may be double bonds or triple bonds.
The later valency of the carbon is fulfilled by the hydrogen atoms. The general formula of the unsaturated hydrocarbon is for alkenes – CnH2n, and for the alkynes is – CnH2n-2.
The unsaturated hydrocarbons are major in three types – alkenes, alkynes, and aromatic compounds. The properties of the aromatic compounds aren’t the same as those of the alkenes and alkynes.
Also, these compounds are comparatively more stable than that of alkenes or alkynes.
The following are the properties or the uses of the unsaturated hydrocarbons –
- To form an unsaturated hydrocarbon, the hydrogen atoms are less in number along with the multiple bonds (double or triple bond).
- The two carbon atoms attached to the unsaturated hydrocarbon form a bond angle of about 120 degrees.
- The most common aromatic hydrocarbon, benzene, forms a 120-degree bond angle with other atoms.
- In any reaction, when water or carbon dioxide is formed, combustion takes place.
- Oxidation reactions are caused by the addition or subtraction of the hydrogen atoms in the reaction.
Main Differences Between Saturated and Unsaturated Hydrocarbons
- Saturated hydrocarbon is the hydrocarbons that are formed with carbon and hydrogen atoms, and the two carbon atoms are bonded by a single bond, whereas comparatively, on the other side, unsaturated hydrocarbon is the hydrocarbons that are also formed with the carbon and hydrogen atoms and the carbon atoms are connected by the multiple bonds, and it may be a double bond or the triple bond.
- Saturated hydrocarbon is of majorly two types, and are – alkanes and cycloalkanes, whereas comparatively, on the other hand, unsaturated hydrocarbons are of majorly three types and they are – alkenes, alkynes, and aromatic compounds.
- The hybridization shown by the saturated hydrocarbons is sp3, whereas comparatively, on the other side, the hybridization shown by the unsaturated hydrocarbons is sp2 for alkenes and sp1 or sp for the alkynes.
- The presence of the number of hydrogen atoms in the saturated compounds is more, while comparatively, on the other hand, the presence of the number of hydrogen atoms in the unsaturated compounds is less.
- The chemical reactivity shown by the saturated compounds is low as if compared, on the other side by the chemical reactivity shown by the unsaturated compound is high.
- The flame test shown by the saturated compounds is the blue flame, while comparatively, on the other side, the flame test shown by the unsaturated compounds is the sooty flame (or the soil-colored flame).
- Examples of saturated hydrocarbons are – alkanes, cycloalkanes, butane, hexane, octane, etc., while comparatively, on the other hand, the examples of unsaturated hydrocarbons are – aromatic compounds, alkynes, and alkene.
- https://www.sciencedirect.com/science/article/abs/pii/S0376738800006232
- https://pubs.acs.org/doi/abs/10.1021/ct300215p
- https://www.frontiersin.org/articles/10.3389/fchem.2014.00075/full
- https://cdnsciencepub.com/doi/abs/10.1139/v71-612

The section on the main differences between saturated and unsaturated hydrocarbons gives a clear overview of distinct chemical characteristics. This is beneficial for students and professionals alike.
The detailed explanations of saturated and unsaturated hydrocarbons and their chemical properties are very beneficial. The information here is presented in a clear and organized manner.
I found the section on the uses of saturated hydrocarbons and unsaturated hydrocarbons to be particularly interesting. It’s fascinating to see how these compounds are applied in various industries.
Absolutely! Understanding their real-world applications helps to appreciate the significance of these compounds in everyday life.
The comparison table provided in the article is particularly helpful for quickly understanding the distinctions between saturated and unsaturated hydrocarbons. Well done!
Nice article! It provides a comprehensive overview of the differences between saturated and unsaturated hydrocarbons, which is essential for understanding the fundamentals of chemistry.
I totally agree! The article explains the concepts very clearly and concisely.
The article has provided a thorough explanation of the chemical reactivity and properties of saturated and unsaturated hydrocarbons. This kind of detailed understanding is crucial in the field of chemistry.
I appreciate the detailed information on the uses of saturated and unsaturated hydrocarbons. It helps to understand the practical significance of these compounds in our daily lives.
This article nicely outlines the chemical properties and differences between saturated and unsaturated hydrocarbons. It’s a great resource for students and anyone interested in chemistry.
The detailed explanations of saturated hydrocarbons, unsaturated hydrocarbons, and their properties are very informative. It’s great to have such clear information in one place.