Chemistry is a subject that revolves around our lives. Calcium is the main component and is available in different forms. The forms vary from Bicarbonate, Carbonate to Hydroxide and Peroxide.
Often people confuse gypsum with lime but they differ in a lot of ways and usage is also different for different products.
Key Takeaways
- Gypsum is a soft sulfate mineral, whereas lime is derived from calcium carbonate.
- Gypsum is primarily used in construction materials, while lime has applications in agriculture, construction, and water treatment.
- Gypsum is less soluble in water than lime, making it more suitable for humid environments.
Gypsum vs Lime
Gypsum is a soft sulfate mineral that contains calcium sulfate dihydrate (CaSO4·2H2O) as its primary component, used in the construction industry for making drywall and plaster. Lime is a calcium-containing mineral that can be derived from limestone or other rock formations and has no sulfur.

Gypsum is the sulphate salt of Calcium. Gypsum is made when the water surrounding sulphur evaporates, and it comes into contact with oxygen.
This results in the formation of sulphate that comes in contact with Calcium and further with water to form calcium sulphate dihydrate. Gypsum is a hard powdery substance that loses water to form the Plaster of Paris.
Lime is another substance that is a salt of Calcium. The salt is acidic because of the presence of carbonate in it.
The formation of lime is due to the perception of the aragonite or calcite that contains dissolved calcium and is either biological or non-biological. Calcium oxide controls the solubility of calcium carbonate.
Comparison Table
| Parameters of Comparison | Gypsum | Lime |
|---|---|---|
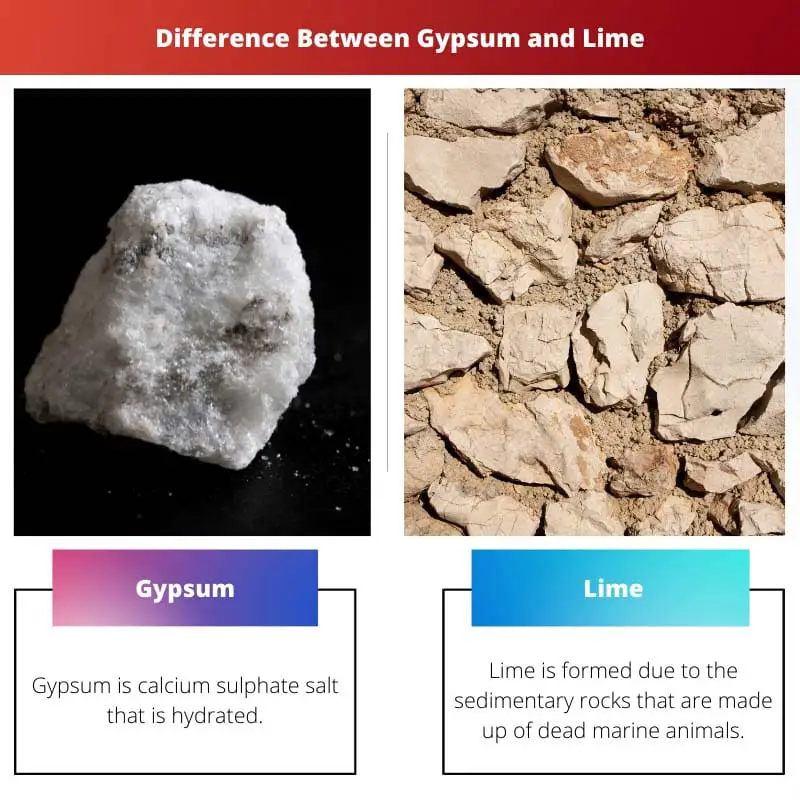
| Definition | Gypsum is calcium sulphate salt that is hydrated. | Lime is formed due to the sedimentary rocks that are made up of dead marine animals. |
| Solubility | Gypsum is soluble in water due to the presence of Sulphate. | Lime is insoluble in water and sediments due to the presence of carbonate in the salt. |
| Acidity | Gypsum is neutral salt and the pH is around 7. | Lime is an acidic salt because the acidic part of the salt is a strong acid. |
| Affect on pH | Gypsum due to being a neutral salt cannot change the pH of the soil. | Lime can change the pH of the soil due to having an acidic group. |
| Crystals | In the process of crystallisation of gypsum, it can grow into larger crystals. | Lime does not grow into larger crystals. |
What is Gypsum?
Gypsum is commonly a Calcium salt and contains sulphate and is known as hydrated calcium sulphate due to the presence of two molecules of water.
The chemical formula of gypsum is CaSO4.2H2O. The formation of gypsum is due to the action of oxygen on the rocks.
The water in the rocks evaporates, and a layer of sulphur is visible. The layer of sulphur combines with the oxygen of the air to form sulphates that attract the elements like Calcium and Magnesium.
The Calcium is in the free form, and the sulphate gets attached to it to form Calcium Sulphate and gains water to form gypsum.
Gypsum has a variety of processes from which it is made. The natural process differs from the industrial process, but it is a useful component.
For the industry, it is used to make Plaster of Paris which is a powdery substance used for a variety of reasons due to its property of becoming hard after drying.
It is formed when Gypsum is heated at a particular temperature and pressure and loses one and a half molecules of water.
Gypsum is a soluble salt due to the presence of a sulphate group. It is also neutral and does not increase the pH of the soil if added in limited quantities.
The salt is neutral because both the acid and base of the elements from where they are obtained are strong.

What is Lime?
Lime is another salt of Calcium with a different acidic group attached to it. The group that is attached is Carbonate, and the chemical name of lime is Calcium Carbonate.
The chemical formula is CaCO3. The formation of lime is due to the sedimentation process.
The formation of lime is underwater due to the formation of sedimentary rock. The sedimentary rock is formed by the dead plants and animals in the ocean, along with the pressure and the action of salty water.
Calcite or aragonite is formed that contains dissolved Calcium and is either biological or non-biological.
The solubility of the salt is quite less in water due to the presence of the carbonate group. The presence of Calcium Oxide also determines the solubility of this salt that controls this.
Calcium Carbonate in the industry is formed by the action of carbon dioxide on calcium oxide, and the excess water is evaporated. On access passing of carbon dioxide, Calcium bicarbonate forms that is soluble in water.
The salt is acidic because it is formed by a weak base and a strong acid. It can also change the pH of the soil to acid and can cause harm to the plants as well as the animals living underneath.

Main Differences Between Gypsum and Lime
- The hydrated calcium sulphate or gypsum has the chemical formula as CaSO4, whereas the sedimented rock at the bottom of the ocean formed due to dead marine life is known as Lime.
- Sulphate dissolves in water easily due to the formation of acid, and therefore gypsum is soluble. In comparison, the presence of carbonate controls the solubility of lime in water and is also in conjugation with Calcium Oxide.
- Gypsum proves to be a neutral salt due to being a mixture of strong acid and base, whereas Lime is said to have acidic properties because of the presence of a strong acid and weak base.
- When gypsum is added to the soil, it does not change the pH, whereas lime is harmful to the soil and causes decay to the plants due to being acidic
- Gypsum forms large crystals and is used in a variety of experiments in chemistry whereas lime does not crystallize but is a very important salt used for testing the formation of carbon dioxide.
- https://acsess.onlinelibrary.wiley.com/doi/abs/10.2136/sssaj1980.03615995004400010010x
- https://ceramics.onlinelibrary.wiley.com/doi/abs/10.1111/j.1151-2916.1987.tb05682.x

Very informative article!
I couldn’t agree more!
This is a great chemistry lesson.
Quite enlightening indeed!
I found the comparison between Gypsum and Lime very interesting.
The details in this article are impressive.
I think this article is oversimplifying the topic of chemistry.
The article is too technical for the average reader to understand.
This article is based on solid scientific research.
A great read for all science enthusiasts!