Molecules and elements are made up of atoms. Atoms are the tiniest unit that cannot be simplified into smaller materials. Elements are derived from the properties of an atom. Atoms form the foundation of molecules and elements.
It is important to make a fine distinction between molecules and elements to study their properties and nature.
Key Takeaways
- An element is a pure substance of atoms with the same number of protons.
- A molecule combines two or more atoms held together by chemical bonds.
- Elements cannot be broken down into simpler substances, while molecules can be broken down into constituent atoms.
Molecule vs Element
The difference between molecules and elements lies in their properties and formation. Molecules consist of two or more atoms forming chemical bonds of similar or dissimilar elements. On the other hand, elements have different formations. They are formed by a single atom. They cannot be brother down into simpler substances as they exist in their pure form.
Molecules are formed by the chemical bonding of two or more atoms. The chemical bonds are formed by the exchange of electrons. When atoms of elements bond with each other, they result in molecules.
The atoms of some elements may be easy to bond with, for example, chlorine, while some may be hard to bond with, for example, argon.
An element consists of a single type of atom. Particles of the elements could be artificial or man-made. Different arrangements of an atom could lead to different versions of that element.
For example, although diamond and graphite are allotropes of carbon, they are quite different in appearance. They have the same chemical structure but dissimilar chemical properties.
Comparison Table
| Parameters of Comparison | Molecule | Element |
|---|---|---|
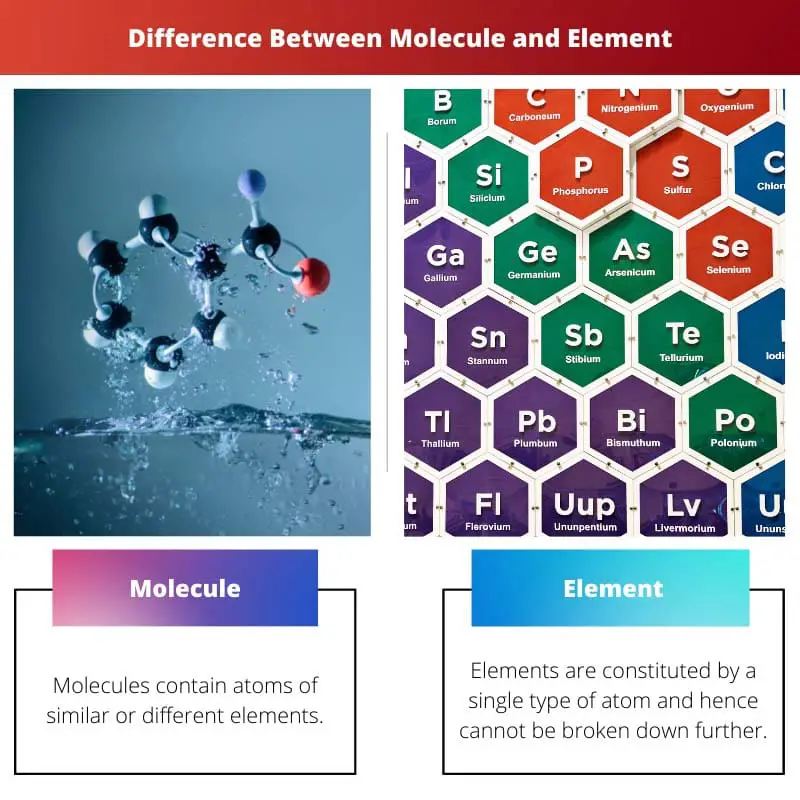
| Atoms | Molecules contain atoms of similar or different elements. | Elements are constituted by a single type of atom and hence cannot be broken down further. |
| Division | It can be divided further using chemical means. | It cannot be divided further by any means. |
| Bonds | They form either ionic or covalent bonds. | They may form various types of bonds depending on stability and electronic configuration. |
| Members | It consists of two or more atoms bonded with each other. | There are presently 115 chemical elements. |
| Examples | Its examples are water, carbon-d-oxide, ozone, etc. | Its examples are nitrogen, copper, zinc, oxygen, etc. |
What is Molecule?
The word molecule refers to atoms that are held together by strong chemical bonds. They vary in size depending on the atoms connected. Some molecules are formed by two similar atoms, and others by complex bondings.
- Two atoms of a similar element: Oxygen(O2), formed by two atoms of oxygen
- Three atoms of a similar element: Ozone(03), formed by three atoms of oxygen
- Complex bonding: Carbon-di-oxide(CO2), formed by bonding of one carbon atom and two oxygen atom
Molecules may be formed by either covalent bonds or ionic bonds. A covalent bond is also known as a shared bond. In covalent bonds, atoms share their electrons, whereas in ionic bonds, an atom donates its electron to another atom.
A single element is not considered a molecule. For example, a single element of oxygen(O) or carbon(C) is not a molecule. Molecules are formed when two or more atoms form a chemical bond.
Some common molecules are ozone, water, glucose, etc.
Molecules are divided based on several categories, some of which are homonuclear and heteronuclear molecules, organic or inorganic molecules, diatomic, triatomic or polyatomic molecules, etc.

What is Element?
Elements are pure substances that cannot be divided or broken down further into simpler matter. They may refer to the atoms having a similar number of protons in their nucleus. They may differ in their masses and neutrons.
When atoms containing similar elements have a distinct number of neutrons, they are referred to as isotopes.
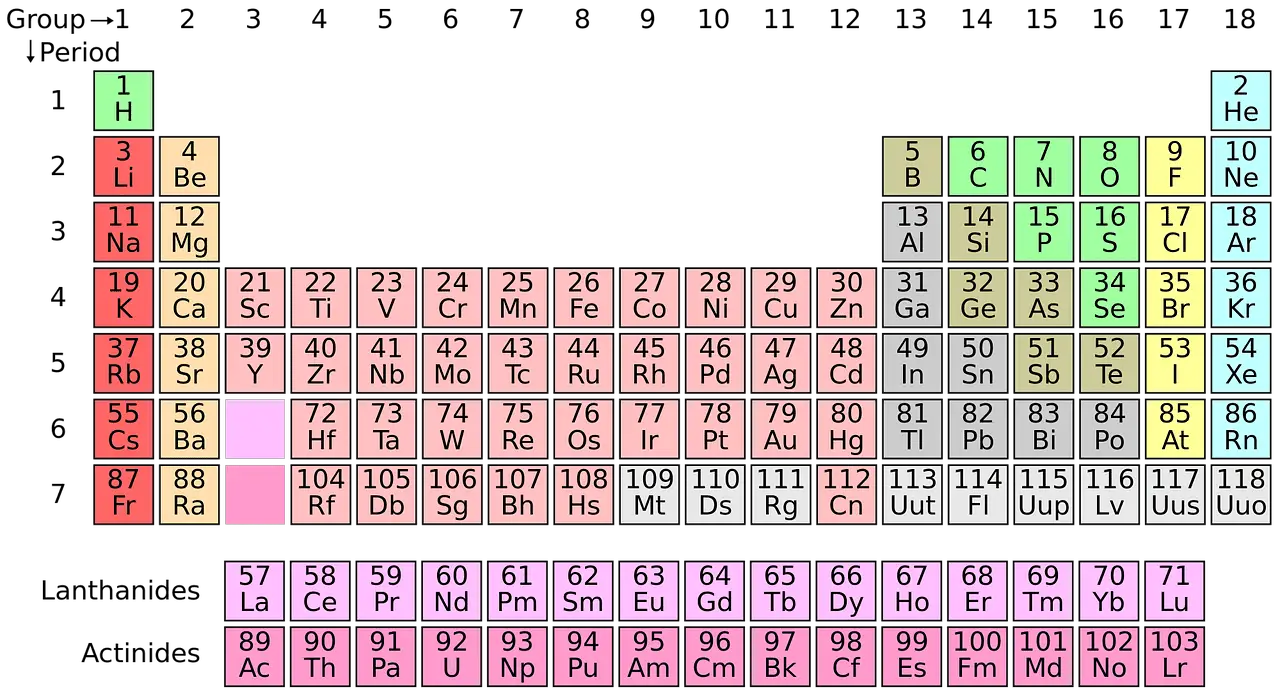
There are 118 elements making their place in the periodic table. In the periodic table, elements are classified based on their characteristics and atomic number.
Each element has its unique atomic symbol, atomic mass, symbol, and electronic arrangement.
There arises a need to classify the elements in the periodic table. It becomes cumbersome to study the properties of each of the 118 elements. So, scientists have developed a way of grouping elements of similar characteristics.
These groupings are done based on four blocks.
- s block: s-block elements are classified into 2 groups. Group 1 elements are named as alkali metals, and group 2 elements are named as alkaline earth metals.
- p block: p-block elements are categorized into metals, nonmetals, and metalloids.
- d block: d-block elements consist of three rows, namely the first, second, and third transition series.
- f block: f-block contains a total of twenty-eight elements that are divided into two equal series. The series of the first fourteen elements is called the lanthanide series. The second set of fourteen elements is called the actinide series.
Main Differences Between Molecule and Element
- A molecule can either be homonuclear or heteronuclear, while an element is referred to as having a unique atomic number.
- Molecules have atoms of similar or different elements, whereas elements contain only similar atoms.
- Molecules and elements differ in their chemical configuration. Molecules can form a specific type of bond, i.e., covalent or ionic bonds. On the other hand, elements can form numerous types of bonds based on electron arrangement.
- The molecule is a substance that can be further divided into smaller substances, whereas elements cannot be further divided by chemical means.
- Examples of Molecules include sugar, glucose, chlorine, acetone, etc. Examples of elements include carbon, hydrogen, helium, etc.
- https://www.sciencedirect.com/science/article/pii/S0166354201002224
- https://aip.scitation.org/doi/abs/10.1063/1.329173

The post provides a comprehensive overview of the characteristics and formations of molecules and elements, offering a detailed understanding of the concepts.
The description of the different blocks in the periodic table is very insightful, providing a thorough understanding of element classifications.
The post effectively captures the distinction between atoms, molecules, and elements, adding clarity to their compositions.
The detailed explanation of the differences between molecules and elements is highly informative and provides a clear understanding of their characteristics.
I appreciate the detailed examples provided, which further clarify the concepts of molecules and elements.
The categorization of elements in the periodic table is explained well, aiding in understanding their grouping based on characteristics.
The categorization of elements in the periodic table is aptly described in the post, offering valuable insights into their classifications.
The detailed explanation of each block in the periodic table makes it easier to comprehend the grouping of elements.
I concur with your assessment, the post effectively outlines the classification of elements based on their characteristics.
The post effectively explains the differences between molecules and elements, offering a comprehensive understanding of their structures and atoms.
The detailed explanation of what constitutes an element as well as a molecule is highly informative and adds depth to the post.
The post discusses the main differences between molecules and elements. It explains the structure and formation of both, and the importance of studying their properties.
Yes, the comparison table provides a clear understanding of the differences between molecules and elements.
I agree, this post is very informative and well-detailed.
The post explains the critical distinctions between molecules and elements. The detailed explanation provides a comprehensive understanding of their composition and structure.
Agreed, the post provides a clear overview of atoms, molecules, and elements.
The examples of molecules and elements further enhance the understanding of the concepts discussed in the post.
The post effectively articulates the differences between molecules and elements, along with the details of their formation and properties.
Spot on, the comparison table and detailed descriptions are very helpful in understanding the concepts.
The detailed comparison table effectively outlines the differences between molecules and elements, providing a thorough understanding of their properties and variations.
I agree, the explanation of the properties and characteristics of molecules and elements is highly detailed and informative.
The classification of elements and their properties are presented in an organized manner, enhancing the comprehensibility of the post.
The detailed comparison between molecules and elements is very insightful, providing a comprehensive understanding of their structures and formations.
The categorization of molecules based on different categories is a valuable addition to the post, enhancing the understanding of the topic.
The difference between covalent and ionic bonds in molecules is well-explained in the post.
The detailed explanation of molecules and elements provides an in-depth understanding of their nature and compositions.