Minerals refer to the elements or chemical compounds which are formed due to various geological processes. These minerals are predominantly crystalline. All minerals have different chemical compositions and properties.
They also have different physical properties. They are used for different purposes. Quartz and Calcite are two such minerals.
Key Takeaways
- Quartz is a silicate mineral with a chemical formula of SiO2, while Calcite is a carbonate mineral with a chemical formula of CaCO3.
- Quartz has a hardness of 7 on the Mohs scale, making it more resistant to scratching, while Calcite has a lower hardness of 3.
- Quartz is piezoelectric, which generates an electric charge when subjected to mechanical stress, whereas Calcite does not possess this property.
Quartz vs Calcite
Quartz is a mineral composed of silicon and oxygen atoms in a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra. Calcite is a carbonate mineral with the chemical formula CaCO3. It is much softer than quartz, with a hardness of only 3 on the Mohs scale.

Quartz is a mineral composed of silica or silicon dioxide. The term has its origin in the German word ‘Quartz. It is a really hard mineral that is commonly found in a hexagonal shape. It is available in different forms.
This crystal also comes in a wide variety of colours.
Calcite is a mineral that is a carbonate mineral with a rhombic cleavage. The term has its origin in the German word ‘Calcit’. It is a moderately hard mineral that is also available in different forms. This crystal’s appearance is white or opaque.
The colour can change if it is charged with impurities.
Comparison Table
| Parameters of Comparison | Quartz | Calcite |
|---|---|---|
| Hardness | In comparison, quartz is approximately four times harder. | In comparison, calcite is approximately four times less hard. |
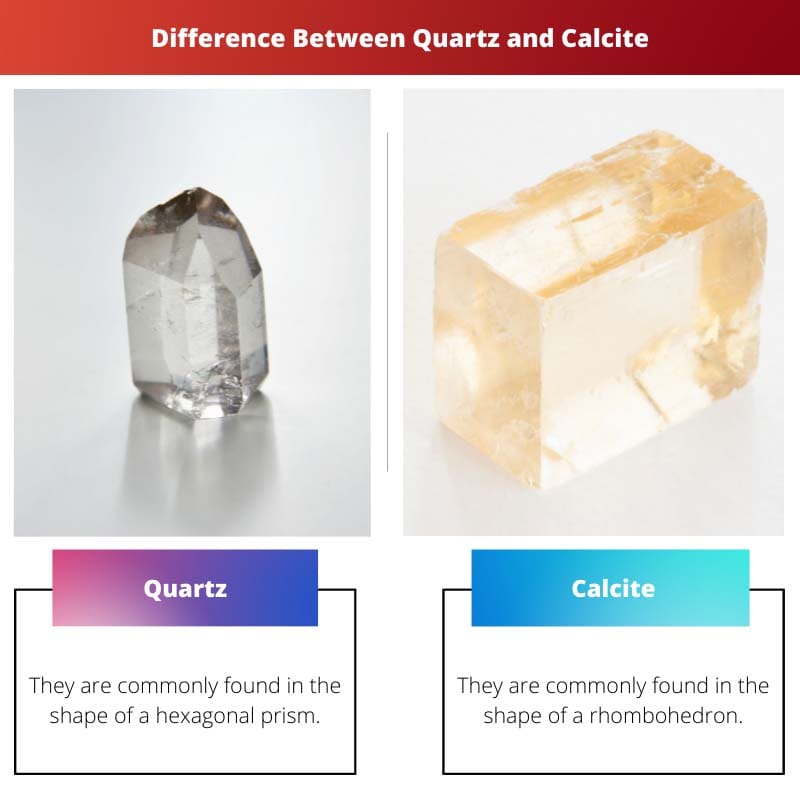
| Shape | They are commonly found in the shape of a hexagonal prism. | They are commonly found in the shape of a rhombohedron. |
| Luster | Their appearance is mostly glassy, or they can also have a vitreous lustre. | It can have a lustre that is vitreous or resinous, or dull. |
| Cleavage | It is not equipped with strong cleavage. | It has a rhombic cleavage. |
| Chemical Composition | In terms of chemical composition, it is a silicon dioxide crystal. | In terms of chemical composition, it is a calcium carbonate mineral. |
What is Quartz?
This term originated from the German word ‘Quarz.’ It is a part of the trigonal crystal system. It is extracted from open-cast mines. Quartz refers to a mineral that is hard and crystalline.
This mineral is composed of silica or silicon dioxide. The overall chemical formula given is SiO2. The second most copious mineral found is Quartz. Its existence comes in two different forms, which are as follows-
- The Normal quartz or the Alpha (α)- quartz
- The High- temperature quartz or the Beta (β)- quartz
Both the quartz are chiral. The Normal quartz or Alpha (α)- quartz can be transformed to High- temperature or Beta (β)- quartz at 573 °C. This transformation comes along with a major change in volume.
This change can lead to ceramic or rock fracture passed through the temperature threshold.
Quartz comes in wide varieties. Most of them are also semi-precious stones. They are one of the most commonly used stones for jewellery making and carvings.
Clear quartz, Amethyst, Citrine, Rose quartz, and Smoky quartz are some popular quartz. It is also believed that these are healing stones. Several people use them to obtain calmness, love, peace, and other things.

What is Calcite?
Calcite refers to a carbonate mineral. This mineral is also the most stable polymorph of calcium carbonate. Aragonite and vaterite are the other polymorphs of calcium carbonate. Aragonite has the ability to transform into calcite.
This can take place over days or even quicker with temperatures above 300 °C. The term calcite originated from the German word ‘Calcit.’ It is also related etymologically to chalk.
This mineral comes in various forms. There are more than 800 identified forms of Calcite. Out of this formsScalenohedra and rhombohedral are the most common ones.
In terms of hardness, it has a defining value of 3 on the Mohs Scale of Mineral Hardness. In terms of appearance, it is opaque or has a white colour. However, it can change its colour if the mineral is charged with certain impurities.
The changed colour is not defined; it may change into any shade, including black.
An optical property termed birefringence is displayed by a calcite crystal. This property is also known as double refraction. Due to this, the objects that are seen through the crystal seem to have doubled.
As it is a carbonate mineral, it can dissolve in acids. This crystal, too, is used for many purposes, including jewellery making and carvings. This mineral, too, is believed to have healing properties.

Main Differences Between Quartz and Calcite
- In comparison, quartz is approximately four times harder. In comparison, calcite is approximately four times less hard.
- Quartz is commonly found in the shape of a hexagonal prism. Calcite is commonly found in the shape of a rhombohedron.
- The appearance of quartz is mostly glassy, or it can also have a vitreous lustre. Calcite can have a lustre that is vitreous or resinous, or dull.
- Quartz is not equipped with strong cleavage. Calcite has a rhombic cleavage.
- Quartz, in terms of chemical composition, is a silicon dioxide crystal. Calcite, in terms of chemical composition, is a calcium carbonate mineral.

I found the explanation about how Calcite can change color when charged with impurities quite interesting.
The comparison table was particularly helpful, it really helped to summarize the main differences between these minerals.
The inclusion of references was a nice touch, it adds credibility to the information presented.
I would have enjoyed a more comical take on the differences between these minerals, I mean, why so serious?
The article went into a lot of depth regarding the chemical composition and properties of quartz and calcite.
The comparisons between quartz and calcite were well detailed, I learned a lot.
I think Quartz is a much more interesting mineral, especially when it comes to its different forms. Very fascinating.
The writer should have focused more on practical uses of these minerals, I found it lacking in that aspect.